Human Papillomavirus Vaccine Market Report
RA08339
Human Papillomavirus Vaccine Market by Type (Tetravalent, Nonavalent, and Bivalent), Disease Indication (Cervical Cancer, Anal Cancer, Vulvar & Vaginal Cancer, Penile Cancer, Oropharyngeal Cancer, and Others), Industry Vertical (Public & Private, Alliances, Government Entities, Physicians, and Others), Regional Outlook (North America, Europe, Asia-Pacific, And LAMEA): Global Opportunity Analysis and Industry Forecast, 2022–2030
Global Human Papillomavirus Vaccine Market Analysis
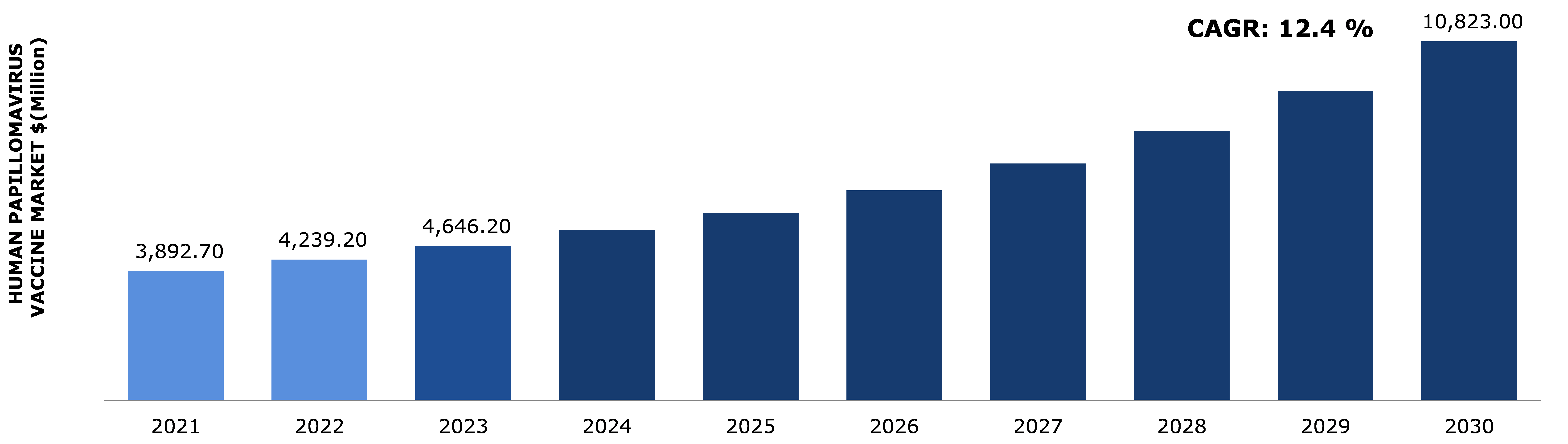
The global human papillomavirus vaccine market is predicted to garner a revenue of $10,823.00 million in the 2022–2030 timeframe, growing from $3,892.70 million in 2021, at a healthy CAGR of 12.4%.
Market Synopsis
Human papillomavirus vaccine market is gaining huge popularity due to increasing cases of human papillomavirus diseases in the population. The introduction of new HPV vaccines, as well as increased initiatives by government and private organizations for early screening and vaccination, is driving the expansion of the human papillomavirus (HPV) vaccine industry. The rising incidences of HPV-related malignancies in the anal, oropharynx, and genital regions, as well as increased demand for HPV vaccinations to help reduce infections, are likely to sustain the market's healthy growth in the growing years. Furthermore, infectious diseases are regarded as the world's greatest killers, providing some of the world's most critical health and security issues. Infectious infections are among the top causes of death worldwide. Scientists from all over the world are currently doing research, exchanging knowledge, expanding laboratory capacity in underdeveloped countries, and constructing global surveillance networks to prevent and control the spread of these diseases by preventative immunizations. Rising government initiatives are expected to boost market expansion throughout the forecast period. Furthermore, some businesses are attempting to ensure a sufficient supply of goods. According to UNICEF estimates from 2018, the UNICEF human papillomavirus (HPV) vaccination obtained around 12.9 million doses over the time period.
However, healthcare costs, on the other hand, are soaring, and the trend is toward prevention. Vaccination costs have climbed from the single digits to the triple digits multiple times in the previous two decades, providing significant challenges for doctors and their patients while also putting a pressure on public health funds.
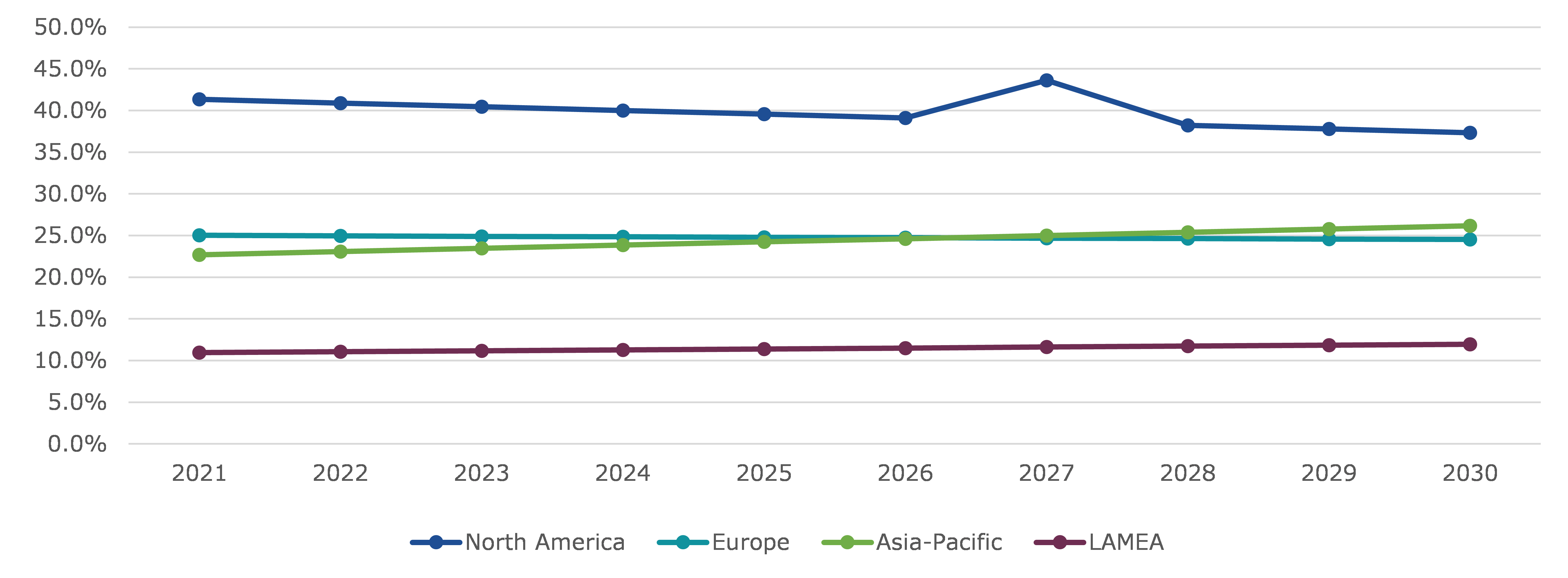
According to regional analysis, the Asia-Pacific human papillomavirus vaccine market accounted for $882.90 million in 2021 and is predicted to grow with a CAGR of 14.2%, in the projected timeframe owing to rising human papillomavirus vaccine demand across the region.
Human Papillomavirus Vaccine Overview
Human papillomavirus (HPV) is a virus that spreads through bodily contact. There are around 100 different forms of HPV, with over 40 of them being transmitted through sexual contact and causing injury to your genital area, face, or mouth. The virus causes HPV spreads through skin-to-skin interaction. The majority of people contract public HPV via direct sexual activity, which involves oral, vaginal, and anal intercourse. Many people have HPV and are unaware of it, which means that one can get it even if their partner is asymptomatic. It also is possible to be multiple HPV kinds. In rare instances, an HPV-positive mother can transmit the infection to her child after birth. When this happens, the kid may get chronic pulmonary papillomatosis, a condition in which they grow HPV-related warts inside their throat or airways. The majority of HPV infections do not result in cancer. but they can increase the risk of cancer in the lower area of the uterus where it connects to the vaginal (cervix). HPV infection has also been linked to cancer of the vagina, vulva, urethra, penis, and back of the neck (oropharyngeal).
COVID-19 Impact on Human Papillomavirus Vaccine Market
The pandemic has created a positive impact on the global human papillomavirus vaccine market. Despite the lockdown and stringent government regulations related to the coronavirus pandemic, most of the healthcare workers are serving their duty and companies are investing heavily in developing the vaccine. Rising need for the demand of HPV vaccine among the population is predicted to drive the market at the time of pandemic. Human papillomavirus is a deadly disease. Vaccine manufactures and many other scientists are continuously working for the development of HPV vaccine along with COVID virus symptoms, which is predicted to boost the market in the estimated period.
Because of the COVID-19 pandemic, most medical, pharmacy, and biotech companies have concentrated on diagnostic kits, safety precautions, and novel coronavirus vaccines & treatments. The majority of the major corporations are focusing their efforts on creating a game-changing vaccine against Novel coronavirus. In addition, medical experts are active in COVID-19-related services. As a result, other sectors of healthcare are suffering. Furthermore, pharmaceutical companies are focusing on COVID-19-related diagnostics, therapeutics, health technologies, and immunizations, as well as evaluating the potential of existing medications. All of these variables are predicted to have a future impact on the global human papillomavirus vaccine industry. Increased investment in vaccine research is expected to push the global human papillomavirus vaccine market in the future years.
Increasing cases of Human Papillomavirus Diseases among the Population is Predicted to Drive the Market in the Estimated Period
Rise in the cases of HPV associated diseases is increasing the demand for HPV vaccines, which is predicted to be the major driving factor of the global human papillomavirus vaccine market in the estimated period. There are only three types of vaccines available in the market: bivalent, quadrivalent, and nonavalent vaccines. All three vaccines are highly effective in preventing infection with virus types 16 and 18, which are responsible for curing 70% of cervical cancer cases. In addition, government and private institutions are taking multiple initiatives for the development and distribution of the HPV vaccine so that the spread of virus can be controlled, which is predicted to drive the market in the estimated timeframe. For instance, the U.S. Food and Drug Administration approved a supplemental application for Gardasil 9 (Human Papillomavirus (HPV) 9-valent Vaccine, Recombinant) expanding the approved use of the vaccine to include women and men aged 27 through 45 years.
Furthermore, several organizations, notably UNICEF, have made attempts to improve vaccine acceptance over the years. The impact of the Pan American Health Organization (PAHO) revolving fund and UNICEF supply division allows vaccinations to be procured at lower prices for diverse countries. PAHO and UNICEF purchase vaccines for roughly 40 states and around 100 countries each year, favorably boosting the adoption rate. Immunization prevents roughly 2-3 million fatalities from diseases such as viral, influenza, tetanus, diphtheria, measles, pertussis, and others. Furthermore, the WHO estimates that improved worldwide immunization coverage might avoid more than 1.5 million deaths. Globally, approximately 116.3 million infants under the age of one year received three doses of the diphtheria-tetanus-pertussis (DTP3) vaccination in 2018. All these aspects are estimated to drive the demand for human papillomavirus vaccine market during 2021-2030.
To know more about global human papillomavirus vaccine market trends, get in touch with our analysts here.
Limited Availability of the Vaccine is Predicted to be the Biggest Restraint for the Human Papillomavirus Vaccine Market in the Projected Timeline
The unavailability of the vaccine to patients at the right time is predicted to be a major drawback for the global human papillomavirus market in the estimated period. There are only two vaccines available in the market. These two vaccines are Gardasil and Cervarix. These two vaccines are also not able to cure the person suffering from HPV under all age groups. For instance, Gardasil is used to cure people from age group 9 to 26 and Cervarix is used in females aged 10-25 to help prevent cervical cancer. Moreover, developing vaccines is an expensive endeavor. The development phase (from in vitro research to commercialization) takes 10 to 20 years and costs between $800 million and $1 billion. All such factors will restrict the market growth.
Majority of Companies are Investing Heavily in Developing New Vaccine which is Predicted to Create more Investment Opportunity for the Market
Various companies are excessively investing in developing other types of HPV vaccine of all age group. The demand for vaccines is increasing at a fast rate where the supply is less. Thus, companies are investing in developing another vaccine against the human papilloma virus with government support, which is predicted to create more investment opportunity market. For instance, in Oct 2018, the U.S. Food and Drug Administration approved a supplemental application for Gardasil 9 for treatment of women and men aged 27 through 45 years.
Furthermore, diseases can occur as a result of the purposeful introduction of pathogens into human, animal, or plant populations for terrorist reasons. Tularemia, anthrax, and smallpox are examples of these diseases. Since the 1970s, chikungunya, avian flu, SARS, MERS, Ebola, swine flu, Zika, and, most recently, COVID-19, caused by a novel coronavirus, SARS-CoV-2, have all been discovered. Experts warn that future pandemics and viruses could be far deadlier than COVID-19. The expanding prevalence of these infectious diseases will ensure a continuous and growing demand for vaccines in the next years. Furthermore, the Introduction of new diseases will result in increased R&D funding initiatives, a robust pipeline, and new vaccination prospects for a diverse variety of population segments.
To know more about global human papillomavirus vaccine market opportunities, get in touch with our analysts here.
Global Human Papillomavirus Vaccine Market, by Type
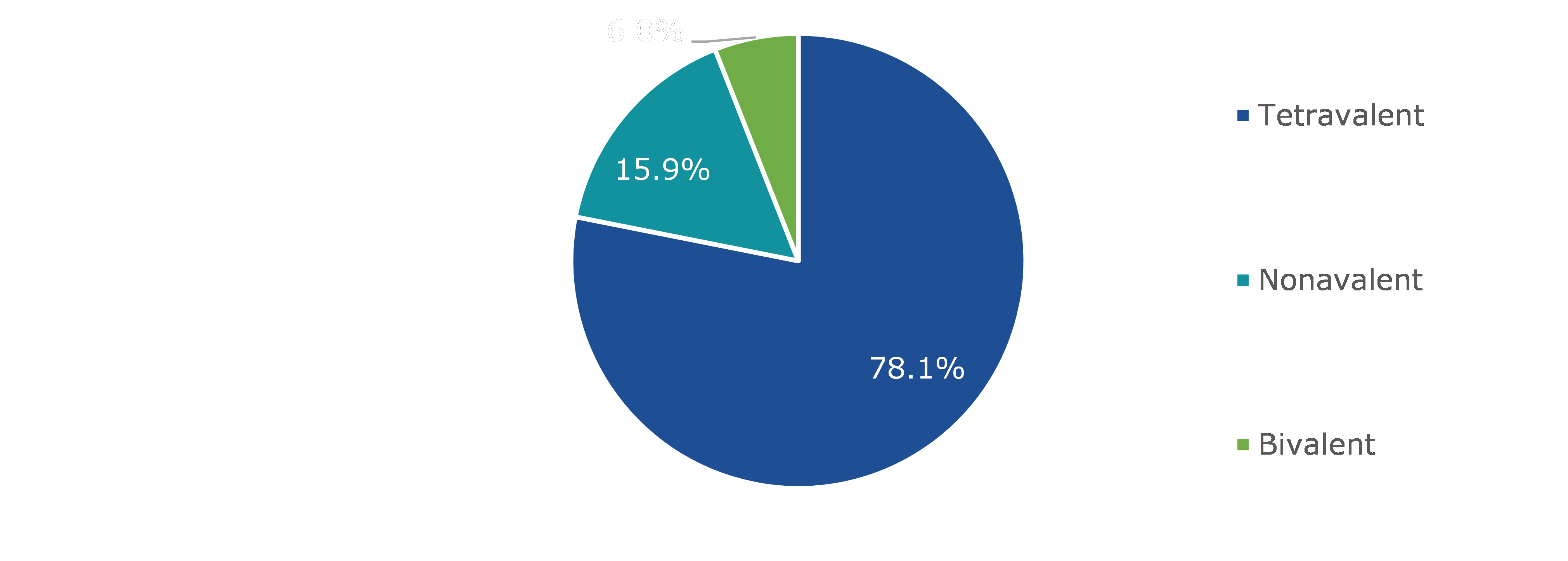
Based on type, the market has been divided into tetravalent, nonavalent, and bivalent. Among these, the tetravalent sub-segment accounted for the highest revenue share in 2021 during the forecast period.
Global Human Papillomavirus Vaccine Market Share, By Type, 2021
Source: Research Dive Analysis
In 2021, the global tetravalent human papillomavirus vaccine market had a dominant market share and generate a revenue of $8,209.20 million by 2030, growing from $3,040.50 million in 2021. Tetravalent human papillomavirus vaccine protects a person from infection by HPV type 6, 11, 16, and 18. These types of human papillomavirus are responsible for 80% of cervical cancers and at least 90% of cases of genital warts globally. The current medicines are based on malware particles (VLPs) generated via HPV surface components. Viral vectors are not infectious since they lack a disease's DNA. They mimic the actual virus, though, and antibodies against the VLPs attack the natural virus as well. VLPs have been shown to be highly immunogenic, causing the body to manufacture antibodies. As a result, vaccines are extremely effective. The vaccines are not required to protect against other sexually transmitted diseases, and they do not treat pre-existing HPV infections. All these factors are estimated to drive the demand for tetravalent human papillomavirus vaccine during the forecast period.
Global Human Papillomavirus Vaccine Market, by Disease Indication
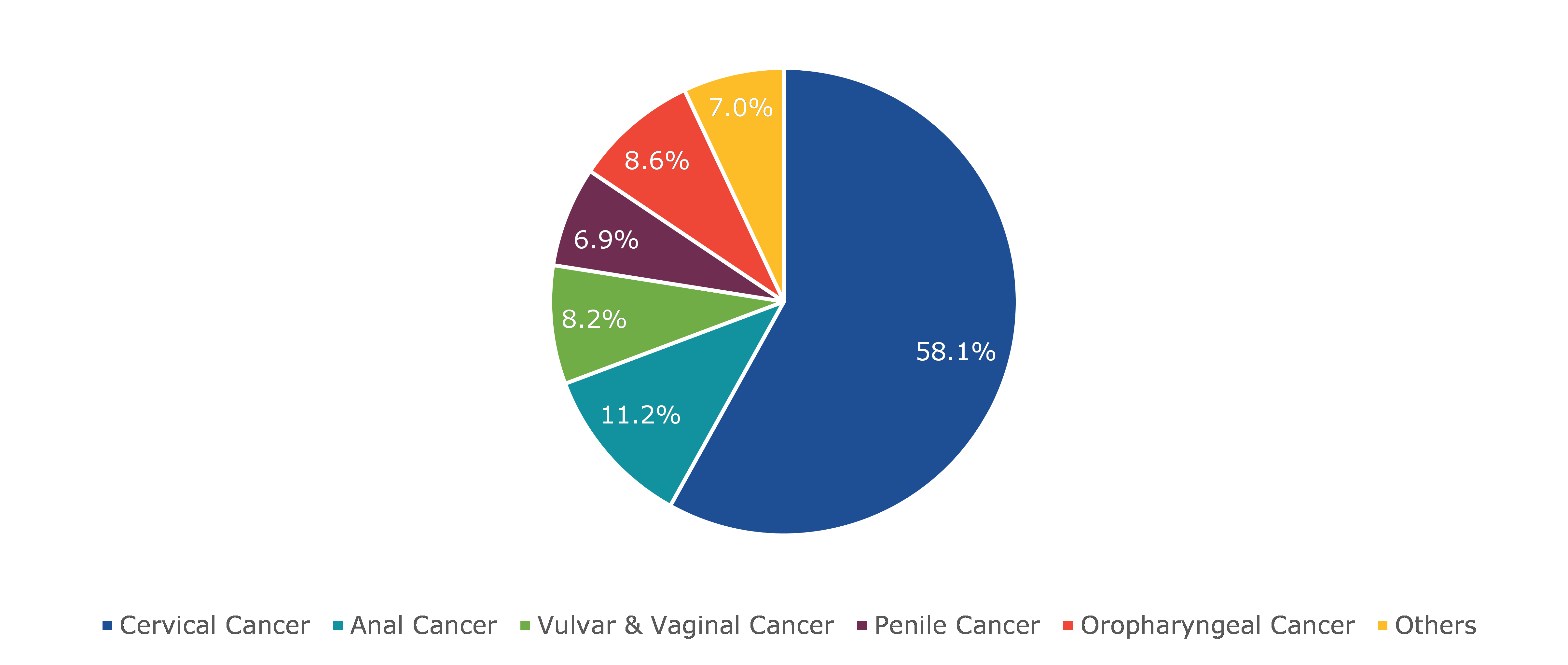
Based on disease indication, the market has been divided into cervical cancer, anal cancer, vulvar & vaginal cancer, penile cancer, oropharyngeal cancer, and others. Among these, the cervical cancer sub-segment accounted for the highest revenue share in 2021 during the forecast period.
Global Human Papillomavirus Vaccine Market Share, By Disease Indication, 2021
Source: Research Dive Analysis
The cervical cancer human papillomavirus vaccine sub-segment is anticipated to dominate the market during the forecast period and generate a revenue of $6,118.00 million by 2030, growing from $2,261.00 million in 2021, with a CAGR of 12.1%. The human papillomavirus is responsible for nearly all occurrences of cervical cancer (HPV). Additionally, in cervical cancer screening, very sensitive and specific molecular assays for identifying high-risk HPV strains from DNA are now available. Furthermore, according to the US National Institute of Health, cervical cancer, which was once one of the most prevalent tumors in the US female population, currently ranks 14th in incidence. However, rates continue to be high in developing countries, where more than 80% of cervical cancer cases are diagnosed. Cervical cancer is the most frequent cancer in women and the second biggest cause of cancer-related death worldwide, accounting for around 300,000 deaths per year, according to the World Health Organization. As the number of cases of cervical cancer rises, so does the market. The rise in demand for cervical cancer human papillomavirus vaccine by consumers has generated significant demand for human papillomavirus vaccine industry.
Global Human Papillomavirus Vaccine Market, by Industry Vertical
Based on industry vertical, the market has been divided into public & private alliances, government entities, physicians, and others. Among these, the public & private alliances sub-segment accounted for the highest market share in 2021 and is estimated to show the fastest growth during 2022-2030.
Global Human Papillomavirus Vaccine Market Share, By Industry Vertical, 2021 and 2030
Source: Research Dive Analysis
The public & private alliances sub-segment is anticipated to have a dominant market share and generate a revenue of $4,396.5 million by 2030, growing from $1,559.7 million in 2021. In most of the countries, public as well as private organizations collaborate for treating infections due to the human papilloma virus. More such initiatives are predicted to drive the sub-segment market in the estimated period. Emerging markets such as India, China, and Southeast Asia present intriguing opportunities for the immunization industry due to their enormous patient populations and rising disposable budgets. This potential for growth has tempted significant market actors to invest in these economies. Various organizations and institutions, such as GAVI, are also attempting to improve vaccine use in disadvantaged countries, such as by providing low-cost vaccines. As a result, these countries are significant growth hotspots in the vaccines market. Another developing market trend is the increased need for low-cost immunizations. Companies are investing in and focusing on developing low-cost vaccinations in order to improve the reach of immunization programmers in these economies. All such factors accelerate the sub-segment growth throughout the forecasting.
Global Human Papillomavirus Vaccine Market, Regional Insights:
The human papillomavirus vaccine market was inspected across North America, Europe, Asia-Pacific, and LAMEA.
Global Human Papillomavirus Vaccine Market Size & Forecast, By Region, 2021-2030 (USD Million)
Source: Research Dive Analysis
North America Region Human Papillomavirus Vaccine Market is Predicted to Create More Investment Opportunity for the Global Market in the Forecast Period
North America region market is predicted to have highest market share in the estimated period. North America region market accounted for $1,609.60 million in 2021 and is predicted to grow with a CAGR of 11.2% in the estimated period. North America is projected to be one of the biggest markets in terms of revenue in the estimated period. Initiatives taken by government for treating people infected with the human papilloma virus is predicted to be the major driving factor for the region market in the estimated period. For instance, in Jan 2021, the U.S. Department of Health and Human Services (HHS), Office of the Assistant Secretary for Health (OASH), and Office on Women's Health launched the HPV VAX NOW campaign with the long-term goal of increasing human papillomavirus (HPV) vaccination rates among young adults aged 18 to 26.
The United States is projected to have the largest HPV vaccine market due to its stronger healthcare infrastructure. Each year, around 14 million new instances of sexually transmitted HPV are recorded in the United States, with at least 79 million people considered to be infected, according to the American Sexual Health Association. In the United States, rising instances are expected to promote market expansion. In 2006, the FDA approved Gardasil as the first vaccine to prevent certain malignancies and diseases caused by HPV. Increased Gardasil sales and value, as well as rapid acceptance of human papillomavirus vaccines throughout the region, will benefit regional growth.
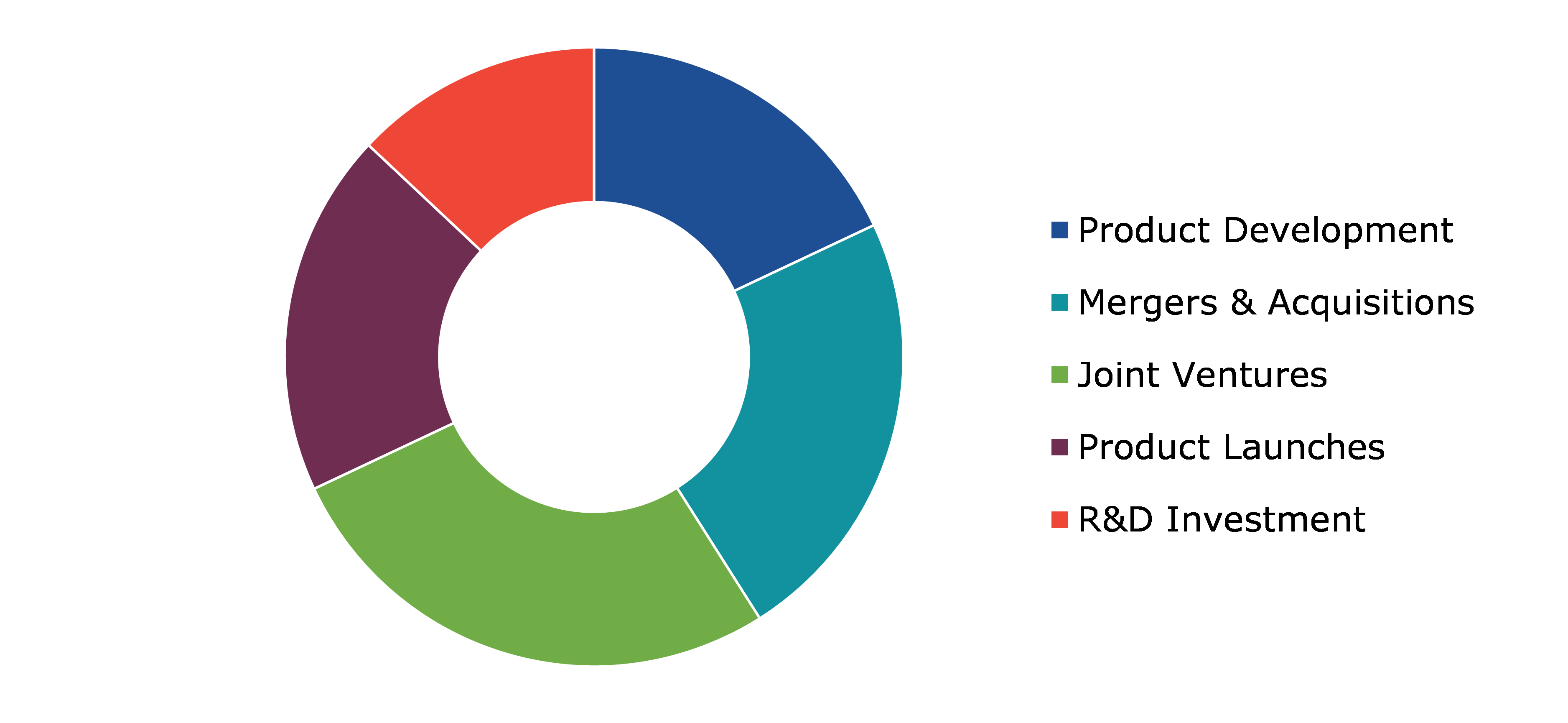
Competitive Scenario in the Global Human Papillomavirus Vaccine Market
Product launches and mergers & acquisitions are common strategies followed by major market players.
Source: Research Dive Analysis
Some of the leading human papillomavirus vaccine market players are GlaxoSmithKline Plc, Johnson & Johnson, Merck & Co., Inc., AstraZeneca Plc, Sanofi S.A., Novartis AG, Serum Institute of India Pvt. Ltd., Bharat Biotech, Inovio Pharmaceuticals, Inc, and Xenetic Biosciences, Inc. among others.
| Aspect | Particulars |
| Historical Market Estimations | 2019-2020 |
| Base Year for Market Estimation | 2021 |
| Forecast Timeline for Market Projection | 2022-2030 |
| Geographical Scope | North America, Europe, Asia-Pacific, LAMEA |
| Segmentation by Type |
|
| Segmentation by Disease Indication |
|
| Segmentation by Industry Vertical |
|
| Key Companies Profiled |
|
Q1. What is the size of the global human papillomavirus vaccine market?
A. The size of the global human papillomavirus vaccine market was over $10,823.0 million in the year 2030, growing from $3,892.7 million in 2021.
Q2. Which are the major companies in the human papillomavirus vaccine market?
A. GlaxoSmithKline Plc and Johnson & Johnson are some of the key players in the global human papillomavirus vaccine market.
Q3. Which region, among others, possesses greater investment opportunities in the near future?
A. The APAC region possesses great investment opportunities for investors to witness the most promising growth in the future.
Q4. What will be the growth rate of the Asia-Pacific human papillomavirus vaccine market?
A. Asia-Pacific human papillomavirus vaccine market is anticipated to grow at 14.2% CAGR during the forecast period.
Q5. What are the strategies opted by the leading players in this market?
A. New product development and strategic partnerships are the key strategies opted by the operating companies in this market.
Q6. Which industries are expected to drive the growth of the human papillomavirus vaccine market in the next 5 years?
A. Cervical cancer human papillomavirus vaccine is expected to drive the growth of the human papillomavirus vaccine market in the next 5 years.
1.Research Methodology
1.1.Desk Research
1.2.Real time insights and validation
1.3.Forecast model
1.4.Assumptions and forecast parameters
1.5.Market size estimation
1.5.1.Top-down approach
1.5.2.Bottom-up approach
2.Report Scope
2.1.Market definition
2.2.Key objectives of the study
2.3.Report overview
2.4.Market segmentation
2.5.Overview of the impact of COVID-19 on Global Human Papillomavirus Vaccine market
3.Executive Summary
4.Market Overview
4.1.Introduction
4.2.Growth impact forces
4.2.1.Drivers
4.2.2.Restraints
4.2.3.Opportunities
4.3.Market value chain analysis
4.3.1.List of raw material suppliers
4.3.2.List of manufacturers
4.3.3.List of distributors
4.4.Innovation & sustainability matrices
4.4.1.Technology matrix
4.4.2.Regulatory matrix
4.5.Porter’s five forces analysis
4.5.1.Bargaining power of suppliers
4.5.2.Bargaining power of consumers
4.5.3.Threat of substitutes
4.5.4.Threat of new entrants
4.5.5.Competitive rivalry intensity
4.6.PESTLE analysis
4.6.1.Political
4.6.2.Economical
4.6.3.Social
4.6.4.Technological
4.6.5.Environmental
4.7.Impact of COVID-19 on Human Papillomavirus Vaccine market
4.7.1.Pre-covid market scenario
4.7.2.Post-covid market scenario
5.Human Papillomavirus Vaccine Market Analysis, by Type
5.1.Overview
5.1.1.Market size and forecast, by Type
5.2.Tetravalent
5.2.1.Key market trends, growth factors, and opportunities
5.2.2.Market size and forecast, by region, 2022-2030
5.2.3.Market share analysis, by country 2020 & 2028
5.3.Nonavalent
5.3.1.Key market trends, growth factors, and opportunities
5.3.2.Market size and forecast, by region, 2022-2030
5.3.3.Market share analysis, by country 2020 & 2028
5.4.Bivalent
5.4.1.Key market trends, growth factors, and opportunities
5.4.2.Market size and forecast, by region, 2022-2030
5.4.3.Market share analysis, by country 2020 & 2028
5.5.Research Dive Exclusive Insights
5.5.1.Market attractiveness
5.5.2.Competition heatmap
6.5.Human Papillomavirus Vaccine Market, by Indication
6.1.Overview
6.1.1.Market size and forecast, by Sales Channel
6.2.Cervical Cancer
6.2.1.Key market trends, growth factors, and opportunities
6.2.2.Market size and forecast, by region, 2022-2030
6.2.3.Market share analysis, by country 2020 & 2028
6.3.Anal Cancer
6.3.1.Key market trends, growth factors, and opportunities
6.3.2.Market size and forecast, by region, 2022-2030
6.3.3.Market share analysis, by country 2020 & 2028
6.4.Vulvar & Vaginal Cancer
6.4.1.Key market trends, growth factors, and opportunities
6.4.2.Market size and forecast, by region, 2022-2030
6.4.3.Market share analysis, by country 2020 & 2028
6.5.Penile Cancer
6.5.1.Key market trends, growth factors, and opportunities
6.5.2.Market size and forecast, by region, 2022-2030
6.5.3.Market share analysis, by country 2020 & 2028
6.6.Oropharyngeal Cancer
6.6.1.Key market trends, growth factors, and opportunities
6.6.2.Market size and forecast, by region, 2022-2030
6.6.3.Market share analysis, by country 2020 & 2028
6.7.Others
6.7.1.Key market trends, growth factors, and opportunities
6.7.2.Market size and forecast, by region, 2022-2030
6.7.3.Market share analysis, by country 2020 & 2028
6.8.Research Dive Exclusive Insights
6.8.1.Market attractiveness
6.8.2.Competition heatmap
7.5.Human Papillomavirus Vaccine Market, by Distribution Channel
7.1.Overview
7.1.1.Market size and forecast, by Sales Channel
7.2.Public and Private Alliances
7.2.1.Key market trends, growth factors, and opportunities
7.2.2.Market size and forecast, by region, 2022-2030
7.2.3.Market share analysis, by country 2020 & 2028
7.3.Government Entities
7.3.1.Key market trends, growth factors, and opportunities
7.3.2.Market size and forecast, by region, 2022-2030
7.3.3.Market share analysis, by country 2020 & 2028
7.4.Physicians
7.4.1.Key market trends, growth factors, and opportunities
7.4.2.Market size and forecast, by region, 2022-2030
7.4.3.Market share analysis, by country 2020 & 2028
7.5.Others
7.5.1.Key market trends, growth factors, and opportunities
7.5.2.Market size and forecast, by region, 2022-2030
7.5.3.Market share analysis, by country 2020 & 2028
7.6.Research Dive Exclusive Insights
7.6.1.Market attractiveness
7.6.2.Competition heatmap
8.5.Human Papillomavirus Vaccine Market, by Region
8.1.North America
8.1.1.U.S.
8.1.2.Market size and forecast, by Type , 2022-2030
8.1.3.Market size and forecast, by Indication , 2022-2030
8.1.4.Market size and forecast, by Distribution Channel , 2022-2030
8.1.5.Canada
8.1.6.Market size and forecast, by Type , 2022-2030
8.1.7.Market size and forecast, by Indication , 2022-2030
8.1.8.Market size and forecast, by Distribution Channel , 2022-2030
8.1.9.Mexico
8.1.10.Market size and forecast, by Type , 2022-2030
8.1.11.Market size and forecast, by Indication , 2022-2030
8.1.12.Market size and forecast, by Distribution Channel , 2022-2030
8.1.13.Research Dive Exclusive Insights
8.1.13.1.Market attractiveness
8.1.13.2.Competition heatmap
8.2.Europe
8.2.1.Germany
8.2.2.Market size and forecast, by Type , 2022-2030
8.2.3.Market size and forecast, by Indication , 2022-2030
8.2.4.Market size and forecast, by Distribution Channel , 2022-2030
8.2.5.UK
8.2.6.Market size and forecast, by Type , 2022-2030
8.2.7.Market size and forecast, by Indication , 2022-2030
8.2.8.Market size and forecast, by Distribution Channel , 2022-2030
8.2.9.France
8.2.10.Market size and forecast, by Type , 2022-2030
8.2.11.Market size and forecast, by Indication , 2022-2030
8.2.12.Market size and forecast, by Distribution Channel , 2022-2030
8.2.13.Spain
8.2.14.Market size and forecast, by Type , 2022-2030
8.2.15.Market size and forecast, by Indication , 2022-2030
8.2.16.Market size and forecast, by Distribution Channel , 2022-2030
8.2.17.Italy
8.2.18.Market size and forecast, by Type , 2022-2030
8.2.19.Market size and forecast, by Indication , 2022-2030
8.2.20.Market size and forecast, by Distribution Channel , 2022-2030
8.2.21.Rest of Europe
8.2.22.Market size and forecast, by Type , 2022-2030
8.2.23.Market size and forecast, by Indication , 2022-2030
8.2.24.Market size and forecast, by Distribution Channel , 2022-2030
8.2.25.Research Dive Exclusive Insights
8.2.25.1.Market attractiveness
8.2.25.2.Competition heatmap
8.3.Asia Pacific
8.3.1.China
8.3.2.Market size and forecast, by Type , 2022-2030
8.3.3.Market size and forecast, by Indication , 2022-2030
8.3.4.Market size and forecast, by Distribution Channel , 2022-2030
8.3.5.Japan
8.3.6.Market size and forecast, by Type , 2022-2030
8.3.7.Market size and forecast, by Indication , 2022-2030
8.3.8.Market size and forecast, by Distribution Channel , 2022-2030
8.3.9.India
8.3.10.Market size and forecast, by Type , 2022-2030
8.3.11.Market size and forecast, by Indication , 2022-2030
8.3.12.Market size and forecast, by Distribution Channel , 2022-2030
8.3.13.Australia
8.3.14.Market size and forecast, by Type , 2022-2030
8.3.15.Market size and forecast, by Indication , 2022-2030
8.3.16.Market size and forecast, by Distribution Channel , 2022-2030
8.3.17.South Korea
8.3.18.Market size and forecast, by Type , 2022-2030
8.3.19.Market size and forecast, by Indication , 2022-2030
8.3.20.Market size and forecast, by Distribution Channel , 2022-2030
8.3.21.Rest of Asia Pacific
8.3.22.Market size and forecast, by Type , 2022-2030
8.3.23.Market size and forecast, by Indication , 2022-2030
8.3.24.Market size and forecast, by Distribution Channel , 2022-2030
8.3.25.Research Dive Exclusive Insights
8.3.25.1.Market attractiveness
8.3.25.2.Competition heatmap
8.4.LAMEA
8.4.1.Brazil
8.4.2.Market size and forecast, by Type , 2022-2030
8.4.3.Market size and forecast, by Indication , 2022-2030
8.4.4.Market size and forecast, by Distribution Channel , 2022-2030
8.4.5.Saudi Arabia
8.4.6.Market size and forecast, by Type , 2022-2030
8.4.7.Market size and forecast, by Indication , 2022-2030
8.4.8.Market size and forecast, by Distribution Channel , 2022-2030
8.4.9.UAE
8.4.10.Market size and forecast, by Type , 2022-2030
8.4.11.Market size and forecast, by Indication , 2022-2030
8.4.12.Market size and forecast, by Distribution Channel , 2022-2030
8.4.13.South Africa
8.4.14.Market size and forecast, by Type , 2022-2030
8.4.15.Market size and forecast, by Indication , 2022-2030
8.4.16.Market size and forecast, by Distribution Channel , 2022-2030
8.4.17.Rest of LAMEA
8.4.18.Market size and forecast, by Type , 2022-2030
8.4.19.Market size and forecast, by Indication , 2022-2030
8.4.20.Market size and forecast, by Distribution Channel , 2022-2030
8.4.21.Research Dive Exclusive Insights
8.4.21.1.Market attractiveness
8.4.21.2.Competition heatmap
9.Competitive Landscape
9.1.Top winning strategies, 2021
9.1.1.By strategy
9.1.2.By year
9.2.Strategic overview
9.3.Market share analysis, 2021
10.Company Profiles
10.1.GlaxoSmithKline Plc
10.1.1.Overview
10.1.2.Business segments
10.1.3.Product portfolio
10.1.4.Financial performance
10.1.5.Recent developments
10.1.6.SWOT analysis
10.2.Johnson & Johnson
10.2.1.Overview
10.2.2.Business segments
10.2.3.Product portfolio
10.2.4.Financial performance
10.2.5.Recent developments
10.2.6.SWOT analysis
10.3.Merck & Co., Inc.,
10.3.1.Overview
10.3.2.Business segments
10.3.3.Product portfolio
10.3.4.Financial performance
10.3.5.Recent developments
10.3.6.SWOT analysis
10.4.AstraZeneca Plc
10.4.1.Overview
10.4.2.Business segments
10.4.3.Product portfolio
10.4.4.Financial performance
10.4.5.Recent developments
10.4.6.SWOT analysis
10.5.Sanofi S.A.
10.5.1.Overview
10.5.2.Business segments
10.5.3.Product portfolio
10.5.4.Financial performance
10.5.5.Recent developments
10.5.6.SWOT analysis
10.6.Novartis AG
10.6.1.Overview
10.6.2.Business segments
10.6.3.Product portfolio
10.6.4.Financial performance
10.6.5.Recent developments
10.6.6.SWOT analysis
10.7.Serum Institute of India Pvt. Ltd.
10.7.1.Overview
10.7.2.Business segments
10.7.3.Product portfolio
10.7.4.Financial performance
10.7.5.Recent developments
10.7.6.SWOT analysis
10.8.Bharat Biotech
10.8.1.Overview
10.8.2.Business segments
10.8.3.Product portfolio
10.8.4.Financial performance
10.8.5.Recent developments
10.8.6.SWOT analysis
10.9.Inovio Pharmaceuticals, Inc
10.9.1.Overview
10.9.2.Business segments
10.9.3.Product portfolio
10.9.4.Financial performance
10.9.5.Recent developments
10.9.6.SWOT analysis
10.10.Xenetic Biosciences, Inc.
10.10.1.Overview
10.10.2.Business segments
10.10.3.Product portfolio
10.10.4.Financial performance
10.10.5.Recent developments
10.10.6.SWOT analysis
11.Appendix
11.1.Parent & peer market analysis
11.2.Premium insights from industry experts
11.3.Related reports
Human Papillomavirus (HPV) vaccine protects against infections with HPV, which is a group of over 200 related viruses, of which over 40 are transmitted by direct sexual contact. Amongst these, two HPV forms cause genital lumps and some other HPV types result in cancers, such as anal, cervical, penile, oropharyngeal, vulvar, and vaginal. Similar to other vaccines that protect against viral infections, HPV vaccines help the body to develop antibodies that can fight against HPV and protect other cells in the body from getting infected.
Genital HPV infection is the most common sexually transmitted infection and is detected in nearly 9–13% of the world’s population and about 6 million people are being infected every year. During adolescence or young adulthood the chances of getting infected by HPV are usually high. HPV infection is deliberated to contribute to nearly 100% cervical cancers, about 80% of anal, and 40–60% of vaginal, penile, and vulvar cancers. Presently, two prophylactic HPV vaccines are commercially obtainable and both are developed from purified L1 structural proteins. Increasing cases of HPV diseases among the population worldwide is boosting the growth of the global human papillomavirus vaccine market.
Why are HPV Vaccines in High Demand?
As per the Centers for Disease Control and Prevention (CDC), HPV is the most commonly occurring sexually transmitted infection in the United States and is associated with health problems including genital warts and cancers. The World Health Organization (WHO) advices to immunize girls in the age group of 9 to 14 years with HPV vaccine, when most of them are not indulged in any sexual activity, to prevent them from getting infected with HPV.
Growing awareness among people about HPV infections and rising cases of HPV diseases in oropharynx, anal, and genital areas across the globe are the major factors boosting the demand for HPV vaccines, thereby driving the growth of the human papillomavirus vaccine market. In addition, governments as well as private institutions are taking up several initiatives for ensuring the availability of the HPV vaccines in order to curb the spread of the virus.
How has the COVID-19 Pandemic Impacted the Human Papillomavirus Vaccine Market?
With the outburst of COVID-19 pandemic the demand for HPV vaccines has surged, thus making a positive impact on the human papillomavirus vaccine industry. According to a research report by Research Dive, the global human papillomavirus vaccine market is anticipated to garner a revenue of $10,823.0 million by 2030, growing at a CAGR of 12.4% from 2022 to 2030. During the pandemic, governments of many regions imposed strict lockdown and implemented several rules to curb the spread of the deadly COVID-19 disease. However, regardless of lockdown restrictions and strict government regulations, the number of COVID-19 infected patients was rising and also most of the healthcare workers were required to perform their duties by risking their lives. Hence, this gave rise to an urgent need to develop effective vaccine, due to which various HPV vaccine manufacturing pharmaceutical companies started investing heavily in research and development of the coronavirus vaccine along with HPV vaccine.
Recent Developments and Key Facts of the Human Papillomavirus Vaccine Market
According to a novel study published in Acta Obstetricia et Gynecologica Scandinavica, a peer-reviewed medical journal covering gynecology, female urology, gynecologic oncology and fertility, HPV vaccine can decrease the chance of high-grade cervical intraepithelial neoplasis (CIN), a condition that can cause cervical cancer.
In addition, in November 2020, the World Health Organization (WHO) has launched a ‘Cervical Cancer Elimination Strategy’ for encouraging all nations to team up and accelerate the elimination of disease with its 3 pronged strategy - 70% screening coverage, 90% HPV vaccine coverage, and 90% access to cervical pre-cancer and cancer treatment.
The key players operating in the global human papillomavirus vaccine market include GlaxoSmithKline Plc, Merck & Co., Inc., Johnson & Johnson, AstraZeneca Plc, Novartis AG, Sanofi S.A., Serum Institute of India Pvt. Ltd., Inovio Pharmaceuticals, Inc., Bharat Biotech, and Xenetic Biosciences, Inc. Besides, several researchers and vaccine manufacturers of the human papillomavirus vaccine market have been taking various initiatives, such as novel developments and researches, strategic partnerships, collaborations, and much more for developing vaccines for HPV. For instance:
- In January 2021, Pregna International Ltd., a leading contraceptive solutions organization, has upgraded its status as a foremost women’s healthcare organization by launching an innovative device which is a cryotherapy device named as CryoPop for fighting cervical cancer.
- In June 2020, Merck known as MSD, a pharmaceutical company, has declared that the U.S. FDA (Food and Drug Administration) has granted approval for an extended indication for GARDASIL 9 for preventing the occurrence of oropharyngeal and other head & neck cancers triggered by HPV types 58, 52, 45, 33, 31, 18, and 16.
Also, researchers are currently studying whether a single dose of HPV vaccine can be effective. A randomized clinical trial is in progress in Costa Rica to examine if a single dose of HPV vaccine is adequate to safeguard against HPV infection.
Future Scope of the Human Papillomavirus Vaccine Industry
As the awareness about prevention of HPV infections is rising, the demand for HPV vaccination is also surging, which is boosting the growth of the global human papillomavirus vaccine market. In addition, growing research & development activities for developing effective HPV vaccines is triggering the industry’s growth. As a final point, the global human papillomavirus vaccine market is soon going to reach newer heights with the development of more efficient vaccines to safeguard the world from human papillomavirus.
Geographically, the North America human papillomavirus vaccine market is estimated to create massive opportunities in the projected timeframe, mainly due to the initiatives taken by government for treating HPV infected people with the human papilloma virus is predicted to be the major driving factor for the region market in the estimated period. For example, in January 2021, the U.S. Department of Health and Human Services (HHS), Office on Women’s Health, and Office of the Assistant Secretary for Health (OASH), launched the HPV VAX NOW campaign with the long-term goal of increasing the rate of human papillomavirus (HPV) vaccination among young adults aged 18 to 26. Besides, the U.S. is projected to have the largest HPV vaccine market owing to its stronger healthcare infrastructure, which is predicted to fortify the growth of the human papillomavirus vaccine market in the near future.
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com