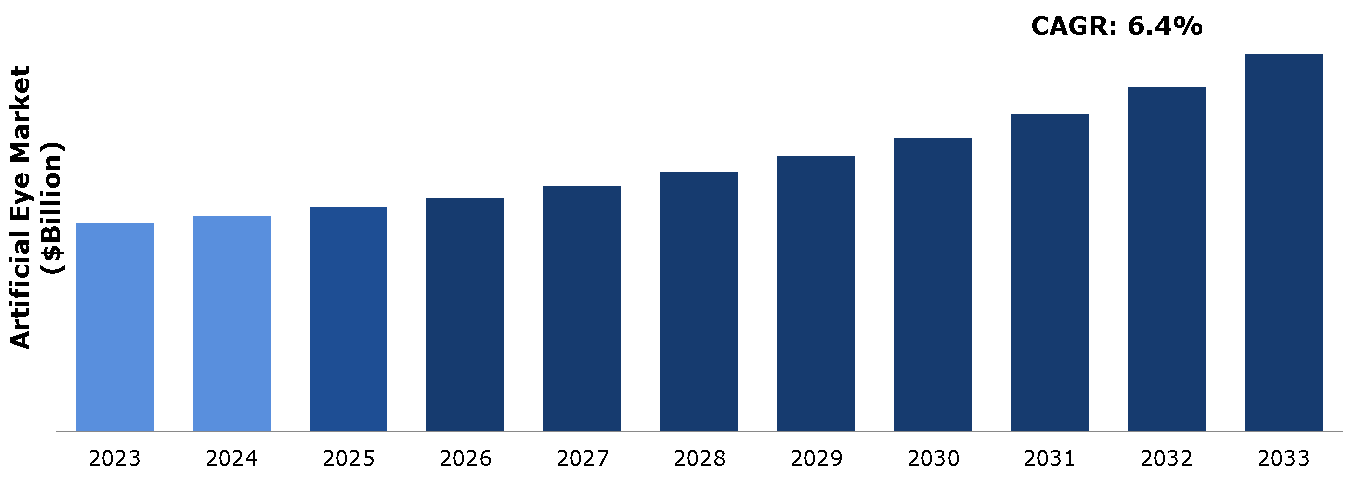Artificial Eye Market Size Share Competitive Landscape And Trend Analysis Report Report
RA00972
Artificial Eye Market Size, Share, Competitive Landscape, and Trend Analysis Report by Product, Technology, End Use, and Region: Global Opportunity Analysis and Industry Forecast, 2024-2033
The artificial eye market was valued at $1.94 billion in 2023 and is estimated to reach $3.52 billion by 2033, exhibiting a CAGR of 6.4% from 2024 to 2033.
Market Definition and Overview
An artificial eye, or ocular prosthesis, is a device designed to replace an absent natural eye. It serves cosmetic purposes, restoring the appearance of a normal eye for those who have lost one due to injury, disease, or congenital conditions. Typically made from acrylic or silicone, it includes a painted iris and pupil to match the patient's other eye. While it does not restore vision, an artificial eye helps maintain facial structure, support eyelids, and is shown to boost the psychological well-being of the individual. Modern advancements focus on improving the prosthesis' fit, comfort, and aesthetic realism.
Key Takeaways
- The artificial eye market study covers 20 countries. The research includes a segment analysis of each country in terms of value ($billion) for the projected period from 2024 to 2033.
- More than 1,500 product literatures, industry releases, annual reports, and other such documents of major long term care industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and assist stakeholders in making informed decisions in order to achieve their most ambitious growth objectives.
Industry Trends
- On July 10, 2023, Eyenuk, a leading expert in medical AI, received approval from the U.S. Food and Drug Administration (FDA) for its groundbreaking technology. This innovation analyzes retinal images to detect symptoms of diabetic retinopathy, representing a significant advancement in the early detection and management of the condition.
- On June 11, 2023, the FDA approved a synthetic tissue substitute developed by CorNeat Vision, an Israeli firm. This pioneering product, named EverPatch, is the first non-degradable synthetic tissue approved globally for eye surgeries. Designed to replace donor tissue and minimize the risk of disease transmission, EverPatch promises safer ophthalmic procedures and heralds a new era in eye surgery.
Market Dynamics
Improving healthcare infrastructure, particularly in emerging economies, facilitates better access to healthcare facilities, fostering heightened awareness, diagnosis, and treatment of various eye conditions. This enhanced accessibility drives a surge in demand for artificial eyes within the global market. As more individuals gain access to quality healthcare services, there's a parallel rise in the need for prosthetic solutions like artificial eyes, catering to those who have experienced eye loss due to injury, disease, or congenital factors. Consequently, the artificial eye market is poised for growth, propelled by advancements in healthcare infrastructure and the expanding reach of medical services worldwide.
One notable restraint in the artificial eye market is the cost associated with prosthetic eye solutions. Despite advancements in technology, these devices can still be expensive, limiting accessibility for certain segments of the population, particularly in regions with lower income levels or inadequate insurance coverage. Additionally, the need for skilled ocularists and specialized facilities for fitting and maintenance adds to the overall expense, posing a barrier to wider adoption.
The growing awareness and acceptance of artificial eyes present a significant opportunity in the market. As availability and advantages of prosthetics expand, alongside diminishing social stigma, more individuals are embracing these solutions. This shift in perception not only fosters a more inclusive society but also creates a larger market demand. With greater acceptance comes increased utilization of artificial eyes, driving innovation and customization within the industry. Manufacturers and developers have the opportunity to capitalize on this trend by offering advanced, aesthetically pleasing, and functional artificial eye options, thus catering to a broader audience and opportunity market growth.
Patent Analysis of Global Artificial Eye Market
Analyzing patents in the artificial eye market provides valuable insights into technological advancements, competitive landscapes, and future trends.
- In February 2023, META PLATFORMS TECHNOLOGIES, LLC launched an artificial eye system. This cutting-edge technology aims to revolutionize visual aids by providing advanced visual capabilities to individuals with impaired vision. The artificial eye system leverages state-of-the-art sensors and imaging technology to enhance sight, offering a significant improvement in quality of life for those affected by vision loss.
- In April 2019, the Xiangya Hospital of Central South University in China introduced an artificial eye composed of an eye table with a porous base, featuring a central hole; a curved artificial eye piece placed atop the table, connected movably via a removable cylinder pin, supporting it through a central groove.
Market Segmentation
The market is segmented into product, technology, end use, and region. On the basis of product, the market is divided into Integrated, semi-integrated, and non-integrated. As per technology, the market is segregated into electronic and mechanical. On the basis of end use, the market is segmented into hospitals, ambulatory surgical centers, and others. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional Market Outlook
The rising prevalence of eye injuries and illnesses is a key driver in the North America artificial eye market. Surgeons and ophthalmologists prefer artificial eyes for transplantation procedures post-injury. With an estimated 2.4 million eye injuries occurring annually in the U.S. alone, there's a significant demand for artificial eye solutions, highlighting the crucial role they play in focus on this healthcare challenge.
- On February 23, 2024, United Ocular announced its acquisition of Eye Prosthetics of Utah, a leading provider known for personalized ocular prosthetics. This strategic acquisition strengthens United Ocular's commitment to assisting individuals dealing with eye loss due to trauma, accidents, or illness. It emphasizes the company's dedication to enhancing quality of life by offering customized solutions tailored to each individual's needs.
- In January 2022, Bionic Vision Technologies (BVT) announced that its Bionic Eye System received Breakthrough Device designation from the FDA in the United States. This achievement represents a significant advancement in artificial eye technology, showcasing the groundbreaking potential of bionic vision technology to revolutionize vision restoration for individuals with visual impairments.
Competitive Landscape
The major players operating in the artificial eye market include Advanced Artificial Eyes, Erickson Laboratories, Second Sight, Studley Ocular Laboratories, F.AD. Müller Söhne GmbH & Co. KG, Irises Unlimited, Ferdinand A. Förster GmbH, Neu Micromed International Pvt. Ltd, and International Prosthetic Eye Center.
Recent Key Strategies and Developments
-
On November 2021, Fraunhofer Technology, a Germany-based company specializing in visual computing research, unveiled innovative technologies that surpass traditional prosthetic production methods. Their Cuttlefish Eye software uses a 3D scan of the eye socket and a color-calibrated image of the healthy eye to create an accurate 3D model of the prosthetic eye. This model is then printed using Fraunhofer's Cuttlefish 3D printing driver on a versatile multicolor, multi-material 3D printer.
- In February 2020, the German company Osmed GmbH introduced "EYEMATE-IO," an advanced artificial eye system. Utilizing cutting-edge materials and design, EYEMATE-IO provides a more natural and adaptable appearance, significantly enhancing patient comfort.
Key Sources Referred
- Annual Reports
- Investor Presentations
- Press Releases
- Artificial Eye technology Pdf
- Research Papers
- D&B Hoovers
- Artificial Eye Investment & Trade Reports
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the artificial eye market segments, current trends, estimations, and dynamics of the artificial eye market analysis from 2024 to 2033 to identify the prevailing artificial eye market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the artificial eye market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global artificial eye market statistics.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global artificial market trends, key players, market segments, application areas, and market growth strategies.
The artificial eye market is witnessing a transformative phase driven by technological advancements and growing demand for realistic and functional prosthetic solutions. This comprehensive report analyzes key market trends, including advancements in materials science, 3D printing, and biotechnology, shaping the industry landscape. It also delves into market dynamics, competitive intelligence, regulatory frameworks, and future growth prospects, providing valuable insights for stakeholders and decision-makers in the healthcare sector.
| Aspect | Particulars |
| Historical Market Estimations | 2021-2022 |
| Base Year for Market Estimation | 2023 |
| Forecast Timeline for Market Projection | 2024-2033 |
| Geographical Scope | North America, Europe, Asia-Pacific, and LAMEA |
| Segmentation by Product |
|
| Segmentation by Technology |
|
|
Segmentation by End Use
|
|
| Key Companies Profiled |
|
1. Research Methodology
1.1. Desk Research
1.2. Real time insights and validation
1.3. Forecast model
1.4. Assumptions and forecast parameters
1.5. Market size estimation
1.5.1. Top-down approach
1.5.2. Bottom-up approach
2. Report Scope
2.1. Market definition
2.2. Key objectives of the study
2.3. Market segmentation
3. Executive Summary
4. Market Overview
4.1. Introduction
4.2. Growth impact forces
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunities
4.3. Market value chain analysis
4.3.1. List of raw material suppliers
4.3.2. List of manufacturers
4.3.3. List of distributors
4.4. Innovation & sustainability matrices
4.4.1. Technology matrix
4.4.2. Regulatory matrix
4.5. Porter’s five forces analysis
4.5.1. Bargaining power of suppliers
4.5.2. Bargaining power of consumers
4.5.3. Threat of substitutes
4.5.4. Threat of new entrants
4.5.5. Competitive rivalry intensity
4.6. PESTLE analysis
4.6.1. Political
4.6.2. Economical
4.6.3. Social
4.6.4. Technological
4.6.5. Legal
4.6.6. Environmental
4.7. Impact of COVID-19 on artificial eye market
4.7.1. Pre-covid market scenario
4.7.2. Post-covid market scenario
5. Artificial Eye Market Analysis, by Product
5.1. Overview
5.2. Integrated
5.2.1. Definition, key trends, growth factors, and opportunities
5.2.2. Market size analysis, by region, 2024-2033
5.2.3. Market share analysis, by country, 2024-2033
5.3. Semi-integrated
5.3.1. Definition, key trends, growth factors, and opportunities
5.3.2. Market size analysis, by region, 2024-2033
5.3.3. Market share analysis, by country, 2024-2033
5.4. Non-integrated
5.4.1. Definition, key trends, growth factors, and opportunities
5.4.2. Market size analysis, by region, 2024-2033
5.4.3. Market share analysis, by country, 2024-2033
5.5. Research Dive Exclusive Insights
5.5.1. Market attractiveness
5.5.2. Competition heatmap
6. Artificial Eye Market Analysis, by Technology
6.1. Overview
6.2. Electronic
6.2.1. Definition, key trends, growth factors, and opportunities
6.2.2. Market size analysis, by region, 2024-2033
6.2.3. Market share analysis, by country, 2024-2033
6.3. Mechanical
6.3.1. Definition, key trends, growth factors, and opportunities
6.3.2. Market size analysis, by region, 2024-2033
6.3.3. Market share analysis, by country, 2024-2033
6.4. Research Dive Exclusive Insights
6.4.1. Market attractiveness
6.4.2. Competition heatmap
7. Artificial Eye Market Analysis, by End Use
7.1. Overview
7.2. Hospital
7.2.1. Definition, key trends, growth factors, and opportunities
7.2.2. Market size analysis, by region, 2024-2033
7.2.3. Market share analysis, by country, 2024-2033
7.3. Ambulatory Surgical Centers
7.3.1. Definition, key trends, growth factors, and opportunities
7.3.2. Market size analysis, by region, 2024-2033
7.3.3. Market share analysis, by country, 2024-2033
7.4. Others
7.4.1. Definition, key trends, growth factors, and opportunities
7.4.2. Market size analysis, by region, 2024-2033
7.4.3. Market share analysis, by country, 2024-2033
7.5. Research Dive Exclusive Insights
7.5.1. Market attractiveness
7.5.2. Competition heatmap
8. Artificial Eye Market, by Region
8.1. North America
8.1.1. U.S.
8.1.1.1. Market size analysis, by Product, 2024-2033
8.1.1.2. Market size analysis, by Technology, 2024-2033
8.1.1.3. Market size analysis, by End Use, 2024-2033
8.1.2. Canada
8.1.2.1. Market size analysis, by Product, 2024-2033
8.1.2.2. Market size analysis, by Technology, 2024-2033
8.1.2.3. Market size analysis, by End Use, 2024-2033
8.1.3. Mexico
8.1.3.1. Market size analysis, by Product, 2024-2033
8.1.3.2. Market size analysis, by Technology, 2024-2033
8.1.3.3. Market size analysis, by End Use, 2024-2033
8.1.4. Research Dive Exclusive Insights
8.1.4.1. Market attractiveness
8.1.4.2. Competition heatmap
8.2. Europe
8.2.1. Germany
8.2.1.1. Market size analysis, by Product, 2024-2033
8.2.1.2. Market size analysis, by Technology, 2024-2033
8.2.1.3. Market size analysis, by End Use, 2024-2033
8.2.2. UK
8.2.2.1. Market size analysis, by Product, 2024-2033
8.2.2.2. Market size analysis, by Technology, 2024-2033
8.2.2.3. Market size analysis, by End Use, 2024-2033
8.2.3. France
8.2.3.1. Market size analysis, by Product, 2024-2033
8.2.3.2. Market size analysis, by Technology, 2024-2033
8.2.3.3. Market size analysis, by End Use, 2024-2033
8.2.4. Spain
8.2.4.1. Market size analysis, by Product, 2024-2033
8.2.4.2. Market size analysis, by Technology, 2024-2033
8.2.4.3. Market size analysis, by End Use, 2024-2033
8.2.5. Italy
8.2.5.1. Market size analysis, by Product, 2024-2033
8.2.5.2. Market size analysis, by Technology, 2024-2033
8.2.5.3. Market size analysis, by End Use, 2024-2033
8.2.6. Rest of Europe
8.2.6.1. Market size analysis, by Product, 2024-2033
8.2.6.2. Market size analysis, by Technology, 2024-2033
8.2.6.3. Market size analysis, by End Use, 2024-2033
8.2.7. Research Dive Exclusive Insights
8.2.7.1. Market attractiveness
8.2.7.2. Competition heatmap
8.3. Asia Pacific
8.3.1. China
8.3.1.1. Market size analysis, by Product, 2024-2033
8.3.2. Market size analysis, by Technology, 2024-2033
8.3.3. Market size analysis, by End Use, 2024-2033
8.3.4. Japan
8.3.4.1. Market size analysis, by Product, 2024-2033
8.3.4.2. Market size analysis, by Technology, 2024-2033
8.3.4.3. Market size analysis, by End Use, 2024-2033
8.3.5. India
8.3.5.1. Market size analysis, by Product, 2024-2033
8.3.5.2. Market size analysis, by Technology, 2024-2033
8.3.5.3. Market size analysis, by End Use, 2024-2033
8.3.6. Australia
8.3.6.1. Market size analysis, by Product, 2024-2033
8.3.6.2. Market size analysis, by Technology, 2024-2033
8.3.6.3. Market size analysis, by End Use, 2024-2033
8.3.7. South Korea
8.3.7.1. Market size analysis, by Product, 2024-2033
8.3.7.2. Market size analysis, by Technology, 2024-2033
8.3.7.3. Market size analysis, by End Use, 2024-2033
8.3.8. Rest of Asia Pacific
8.3.8.1. Market size analysis, by Product, 2024-2033
8.3.8.2. Market size analysis, by Technology, 2024-2033
8.3.8.3. Market size analysis, by End Use, 2024-2033
8.3.9. Research Dive Exclusive Insights
8.3.9.1. Market attractiveness
8.3.9.2. Competition heatmap
8.4. LAMEA
8.4.1. Brazil
8.4.1.1. Market size analysis, by Product, 2024-2033
8.4.1.2. Market size analysis, by Technology, 2024-2033
8.4.1.3. Market size analysis, by End Use, 2024-2033
8.4.2. Saudi Arabia
8.4.2.1. Market size analysis, by Product, 2024-2033
8.4.2.2. Market size analysis, by Technology, 2024-2033
8.4.2.3. Market size analysis, by End Use, 2024-2033
8.4.3. UAE
8.4.3.1. Market size analysis, by Product, 2024-2033
8.4.3.2. Market size analysis, by Technology, 2024-2033
8.4.3.3. Market size analysis, by End Use, 2024-2033
8.4.4. South Africa
8.4.4.1. Market size analysis, by Product, 2024-2033
8.4.4.2. Market size analysis, by Technology, 2024-2033
8.4.4.3. Market size analysis, by End Use, 2024-2033
8.4.5. Rest of LAMEA
8.4.5.1. Market size analysis, by Product, 2024-2033
8.4.5.2. Market size analysis, by Technology, 2024-2033
8.4.5.3. Market size analysis, by End Use, 2024-2033
8.4.6. Research Dive Exclusive Insights
8.4.6.1. Market attractiveness
8.4.6.2. Competition heatmap
9. Competitive Landscape
9.1. Top winning strategies, 2023
9.1.1. By strategy
9.1.2. By year
9.2. Strategic overview
9.3. Market share analysis, 2023
10. Company Profiles
10.1. Advanced Artificial Eyes
10.1.1. Overview
10.1.2. Business segments
10.1.3. Product portfolio
10.1.4. Financial performance
10.1.5. Recent developments
10.1.6. SWOT analysis
10.2. International Prosthetic Eye Center
10.2.1. Overview
10.2.2. Business segments
10.2.3. Product portfolio
10.2.4. Financial performance
10.2.5. Recent developments
10.2.6. SWOT analysis
10.3. Erickson Laboratories
10.3.1. Overview
10.3.2. Business segments
10.3.3. Product portfolio
10.3.4. Financial performance
10.3.5. Recent developments
10.3.6. SWOT analysis
10.4. F.AD. Müller Söhne GmbH & Co. KG
10.4.1. Overview
10.4.2. Business segments
10.4.3. Product portfolio
10.4.4. Financial performance
10.4.5. Recent developments
10.4.6. SWOT analysis
10.5. Second Sight
10.5.1. Overview
10.5.2. Business segments
10.5.3. Product portfolio
10.5.4. Financial performance
10.5.5. Recent developments
10.5.6. SWOT analysis
10.6. Ferdinand A. Förster GmbH
10.6.1. Overview
10.6.2. Business segments
10.6.3. Product portfolio
10.6.4. Financial performance
10.6.5. Recent developments
10.6.6. SWOT analysis
10.7. Studley Ocular Laboratories
10.7.1. Overview
10.7.2. Business segments
10.7.3. Product portfolio
10.7.4. Financial performance
10.7.5. Recent developments
10.7.6. SWOT analysis
10.8. Irises Unlimited
10.8.1. Overview
10.8.2. Business segments
10.8.3. Product portfolio
10.8.4. Financial performance
10.8.5. Recent developments
10.8.6. SWOT analysis
10.9. Neu Micromed International Pvt. Ltd
10.9.1. Overview
10.9.2. Business segments
10.9.3. Product portfolio
10.9.4. Financial performance
10.9.5. Recent developments
10.9.6. SWOT analysis
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com