Macular Degeneration Treatment Market Size Share Competitive Landscape And Trend Analysis Report Report
RA00783
Macular Degeneration Treatment Market Size, Share, Competitive Landscape, and Trend Analysis Report by Type, Stage of Disease, Route of Administration, End User, and Region: Global Opportunity Analysis and Industry Forecast, 2024-2033
The macular degeneration treatment market was valued at $14.62 billion in 2023 and is estimated to reach $31.18 billion by 2033, exhibiting a CAGR of 7.9% from 2024 to 2033.
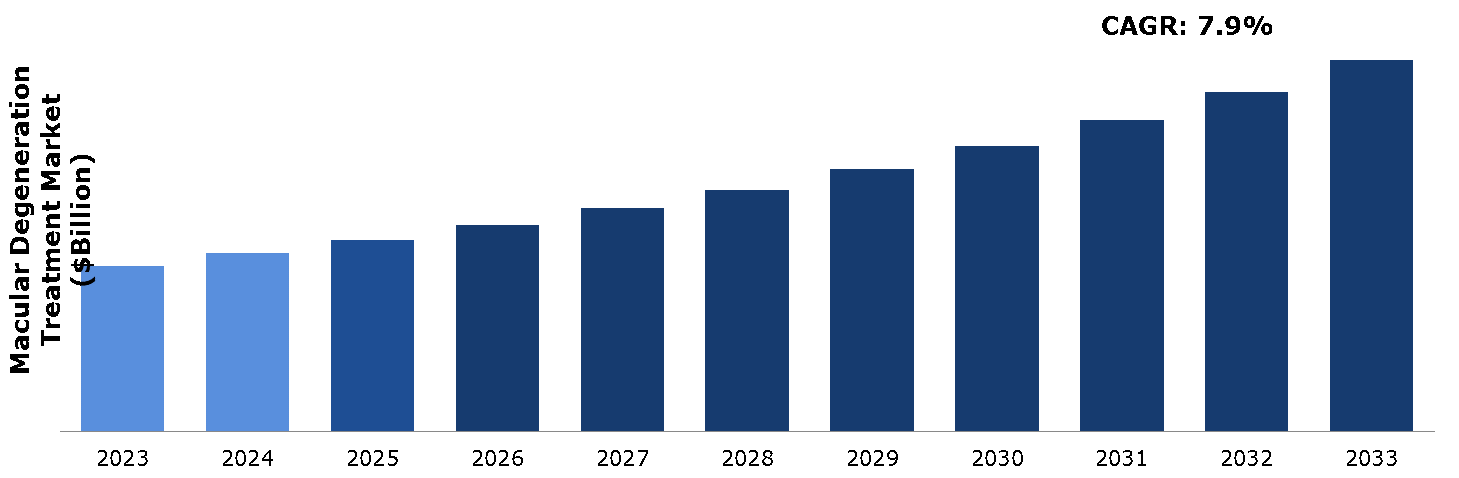
Overview of Macular Degeneration
Macular degeneration, a major cause of vision impairment, arises from the gradual deterioration of the central region of the retina, known as the macula. This vital area is responsible for sharp, detailed vision crucial for activities like reading, driving, and recognizing faces or colors. As the condition progresses through its three stages, symptoms may vary. Early age-related macular degeneration (AMD) often goes unnoticed, detectable only through routine eye examinations where yellow deposits beneath the retina indicate potential trouble. In the intermediate stage, some vision loss occurs, though symptoms may not be apparent. However, in its advanced form, AMD can lead to significant visual impairment, potentially resulting in complete loss of central vision. Timely detection and management play major roles in mitigating the impact of this extreme health condition.
Key Takeaways
- The macular degeneration treatment market study covers 20 countries. The research includes a segment analysis of each country in terms of value ($billion) for the projected period from 2024 to 2033.
- More than 1,500 product literatures, industry releases, annual reports, and other such documents of major macular degeneration treatment industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach intends to provide a balanced view of global markets and assist stakeholders in making informed decisions in order to achieve their most ambitious growth objectives.
Industry Trends
- In June 2022, Roche Canada received Health Canada's approval for VABYSMO (faricimab injection), marking a development in treating neovascular (wet) age-related macular degeneration (AMD) and diabetic macular edema (DME). This innovative therapy targets both VEGF-A and Ang-2, addressing crucial factors in vascular instability linked to vision loss. As the first of its kind in Canada, VABYSMO offers a dual mechanism of action. With this authorization, Canadians suffering with these sight-threatening conditions now have access to the treatment option.
-
In December 2023, The FDA approved Syfovre (pegcetacoplan) and Izervay (avacincaptad pegol) as treatments for geographic atrophy (GA), a severe form of dry age-related macular degeneration (AMD). These drugs focus on regulating the immune system's complement pathway. Their approval marks a significant milestone in combating a condition. Developed by private companies, their achievement owes much to the National Eye Institute's (NEI) extensive grant support, fueling research across various campuses nationwide. This collaborative effort highlights the connection of public and private sectors for medical advancement to address critical unmet needs in healthcare.
Key Market Dynamics
With the global elderly population on the rise, there is a surging need for effective solutions to age-related eye diseases. Moreover, the escalating number of individuals diagnosed with retinal disorders highlights the urgency for innovative treatments. This convergence of demographic trends and medical necessity is driving significant advancements in macular degeneration therapies.
Moreover, complications in healthcare related to macular degeneration treatment pose significant hurdles to its advancement. Despite medical advancements, the emergence of complications often impedes progress in effectively treating this condition. These complications range from adverse reactions to treatment methods to challenges in access and affordability. Such barriers hinder the development of more efficient and accessible treatments for macular degeneration, limiting the prospects for improving patient outcomes and quality of life.
Public Policy Analysis
Effective from October 1, 2023, UnitedHealthcare Commercial and Individual Exchange's Medical Policy 2023T0404Z has established that the Implantable Miniature Telescope (IMT) is proven medically necessary for treating individuals with end-stage age-related macular degeneration (AMD) when used in accordance with FDA guidelines. Additionally, home visual field monitoring, such as ForeseeHome, is deemed medically necessary for detecting AMD-associated choroidal neovascularization (CNV) under specific conditions. These conditions include individuals at risk for developing CNV who have bilateral large drusen or large drusen in one eye and advanced AMD in the other, best corrected visual acuity of 20/60 or better, the ability to operate the device, and no medial opacities or other retinal disorders like diabetic retinopathy. These measures aim to provide early detection and effective treatment for individuals at risk of severe vision loss due to AMD.
Market Segmentation
The macular degeneration treatment market is segmented into type, stage of disease, route of administration, end user, and region. On the basis of type, the market is segmented into dry age-related macular degeneration, and wet age-related macular degeneration. As per stage of disease, the market is divided into early stage, intermediate stage, and late stage. By route of administration, the market is segregated on the basis of intravenous and intravitreal. On the basis of end user, the market is segmented into hospitals and clinics, ambulatory surgical centers, and others. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional Market Outlook
Europe is poised for a surge in demand for macular degeneration treatment. With advancements in healthcare and an aging population, the demand for effective interventions against this vision-threatening condition is steadily increasing. This anticipated rise reflects not only the prevalence of macular degeneration but also the growing awareness and access to medical care across Europe. As research continues to unveil new treatment modalities and therapies, healthcare systems are advancing to meet the rising demand, ensuring that individuals affected by macular degeneration receive timely and effective care to preserve their vision and quality of life.
Izervay (avacincaptad pegol), cleared by the U.S. FDA in August 203, is the second drug approved for use in combating the progression of a late-stage disease. Formerly recognized as Zimura, it targets complement C5 protein, suspected of exacerbating the condition. Clinical trials showcased its efficacy in reducing disease advancement. This approval marks a significant step in the battle against the disease, offering hope to patients facing its adverse effects. With its unique mechanism of action, Izervay presents a reliable treatment for managing and potentially slowing down the deterioration associated with this condition.
In June 2023, Alimera Sciences expanded its commercialization rights for Yutiq, acquiring exclusive global rights, except for specific regions. The acquisition from EyePoint Pharmaceuticals includes rights for treating chronic noninfectious uveitis in the posterior segment of the eye. With this deal, Alimera now holds sole authority over Yutiq's distribution worldwide, excluding China, Hong Kong, Taiwan, Macau, South Korea, and Southeast Asia. EyePoint retains its licensing agreement with Ocumension Therapeutics for these regions. This move signifies Alimera's strategic expansion in the ophthalmology market, bolstering its portfolio and potentially broadening patient access to this innovative treatment.
Competitive Landscape
The major players operating in the macular degeneration treatment market include REGENXBIO Inc., Panoptica, Regeneron, Bausch Health Companies Inc., Novartis AG, F. Hoffmann-La Roche AG, Pfizer Inc., Thermo Fischer Scientific, Inc, Astellas Pharma Inc., and Aerie Pharmaceutical Inc.
Other players in macular degeneration treatment market includes Santen Pharmaceutical Co., Ltd. and Amgen Inc..
Recent Key Strategies and Developments
- In April 2023, Astellas entered into a definitive agreement to acquire Iveric Bio, with Astellas' subsidiary, Berry Merger Sub, Inc., set to purchase all outstanding shares for approximately US$5.9 billion. This acquisition positions Iveric Bio as an indirectly wholly-owned subsidiary of Astellas. The purchase price offers a substantial premium, standing at 64% above Iveric Bio's unaffected closing share price of US$24.33 on March 31, 2023, and 75% above its 30-day volume-weighted average price as of the same date. This strategic move highlights Astellas' commitment to expansion and investment in innovative biopharmaceutical endeavors.
- In December 2021, Novartis completed the acquisition of Gyroscope Therapeutics, a UK-based company in ocular gene therapy. This move marks a significant stride in addressing geographic atrophy (GA), an advanced form of dry age-related macular degeneration (AMD) with no approved treatments. Gyroscope's GT005, an investigational gene therapy, targets GA by rebalancing the overactive complement system, crucial for immune response, through increased CFI protein production. Administered under the retina, GT005 holds promise as a one-time intervention for GA secondary to AMD, potentially halting progressive vision loss. This acquisition highlights Novartis's commitment to pioneering solutions for unmet needs in retinal diseases.
Key Sources Referred
- International Agency for the Prevention of Blindness (IAPB)
- Macular Degeneration Association (MDA)
- American Macular Degeneration Foundation (AMDF)
- Association for Research in Vision and Ophthalmology (ARVO)
- F. Hoffmann-La Roche AG
- Regeneron
- Astellas Pharma Inc.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the macular degeneration treatment market segments, current trends, estimations, and dynamics of the macular degeneration treatment market analysis from 2023 to 2033 to identify the prevailing macular degeneration treatment market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the macular degeneration treatment market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global macular degeneration treatment market statistics.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global macular degeneration treatment market trends, key players, market segments, application areas, and market growth strategies.
The market study comprises of all latest technological advancements, including the latest market development by major players operating in the market. Furthermore, the study also comprises detailed public policy analysis.
| Aspect | Particulars |
| Historical Market Estimations | 2021-2022 |
| Base Year for Market Estimation | 2023 |
| Forecast Timeline for Market Projection | 2024-2033 |
| Geographical Scope | North America, Europe, Asia-Pacific, and LAMEA |
| Segmentation by Type |
|
| Segmentation by Stage of Disease |
|
| Segmentation by Route of Administration |
|
| Segmentation by End User |
|
| Key Companies Profiled |
|
1. Research Methodology
1.1. Desk Research
1.2. Real time insights and validation
1.3. Forecast model
1.4. Assumptions and forecast parameters
1.5. Market size estimation
1.5.1. Top-down approach
1.5.2. Bottom-up approach
2. Report Scope
2.1. Market definition
2.2. Key objectives of the study
2.3. Market segmentation
3. Executive Summary
4. Market Overview
4.1. Introduction
4.2. Growth impact forces
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunities
4.3. Market value chain analysis
4.3.1. List of service providers
4.4. Innovation & sustainability matrices
4.4.1. Technology matrix
4.4.2. Patent matrix
4.4.3. Regulatory matrix
4.5. Porter’s five forces analysis
4.5.1. Bargaining power of suppliers
4.5.2. Bargaining power of consumers
4.5.3. Threat of substitutes
4.5.4. Threat of new entrants
4.5.5. Competitive Rivalry Intensity
4.6. PESTLE analysis
4.6.1. Political
4.6.2. Economical
4.6.3. Social
4.6.4. Technological
4.6.5. Legal
4.6.6. Environmental
5. Macular Degeneration Treatment Market Analysis, by Type
5.1. Overview
5.2. Dry Age-Related Macular Degeneration
5.2.1. Definition, key trends, growth factors, and opportunities
5.2.2. Market size analysis, by region, 2023-2033
5.2.3. Market share analysis, by country, 2023-2033
5.3. Wet Age-Related Macular Degeneration
5.3.1. Definition, key trends, growth factors, and opportunities
5.3.2. Market size analysis, by region, 2023-2033
5.3.3. Market share analysis, by country, 2023-2033
5.4. Research Dive Exclusive Insights
5.4.1. Market attractiveness
5.4.2. Competition heatmap
6. Macular Degeneration Treatment Market Analysis, by Stage of Disease
6.1. Overview
6.2. Early Stage
6.2.1. Definition, key trends, growth factors, and opportunities
6.2.2. Market size analysis, by region, 2023-2033
6.2.3. Market share analysis, by country, 2023-2033
6.3. Intermediate Stage
6.3.1. Definition, key trends, growth factors, and opportunities
6.3.2. Market size analysis, by region, 2023-2033
6.3.3. Market share analysis, by country, 2023-2033
6.4. Late Stage
6.4.1. Definition, key trends, growth factors, and opportunities
6.4.2. Market size analysis, by region, 2023-2033
6.4.3. Market share analysis, by country, 2023-2033
6.5. Research Dive Exclusive Insights
6.5.1. Market attractiveness
6.5.2. Competition heatmap
7. Macular Degeneration Treatment Market Analysis, by Route of Administration
7.1. Overview
7.2. Intravenous
7.2.1. Definition, key trends, growth factors, and opportunities
7.2.2. Market size analysis, by region, 2023-2033
7.2.3. Market share analysis, by country, 2023-2033
7.3. Intravitreal
7.3.1. Definition, key trends, growth factors, and opportunities
7.3.2. Market size analysis, by region, 2023-2033
7.3.3. Market share analysis, by country, 2023-2033
7.4. Research Dive Exclusive Insights
7.4.1. Market attractiveness
7.4.2. Competition heatmap
8. Macular Degeneration Treatment Market Analysis, by End User
8.1. Overview
8.2. Hospitals and Clinics
8.2.1. Definition, key trends, growth factors, and opportunities
8.2.2. Market size analysis, by region, 2023-2033
8.2.3. Market share analysis, by country, 2023-2033
8.3. Ambulatory Surgical Centers
8.3.1. Definition, key trends, growth factors, and opportunities
8.3.2. Market size analysis, by region, 2023-2033
8.3.3. Market share analysis, by country, 2023-2033
8.4. Others
8.4.1. Definition, key trends, growth factors, and opportunities
8.4.2. Market size analysis, by region, 2023-2033
8.4.3. Market share analysis, by country, 2023-2033
8.5. Research Dive Exclusive Insights
8.5.1. Market attractiveness
8.5.2. Competition heatmap
9. Macular Degeneration Treatment Market, by Region
9.1. North America
9.1.1. U.S.
9.1.1.1. Market size analysis, by Type, 2023-2033
9.1.1.2. Market size analysis, by Stage of Disease, 2023-2033
9.1.1.3. Market size analysis, by Route of Administration, 2023-2033
9.1.1.4. Market size analysis, by End-user, 2023-2033
9.1.2. Canada
9.1.2.1. Market size analysis, by Type, 2023-2033
9.1.2.2. Market size analysis, by Stage of Disease, 2023-2033
9.1.2.3. Market size analysis, by Route of Administration, 2023-2033
9.1.2.4. Market size analysis, by End-user, 2023-2033
9.1.3. Mexico
9.1.3.1. Market size analysis, by Type, 2023-2033
9.1.3.2. Market size analysis, by Stage of Disease, 2023-2033
9.1.3.3. Market size analysis, by Route of Administration, 2023-2033
9.1.3.4. Market size analysis, by End-user, 2023-2033
9.1.4. Research Dive Exclusive Insights
9.1.4.1. Market attractiveness
9.1.4.2. Competition heatmap
9.2. Europe
9.2.1. Germany
9.2.1.1. Market size analysis, by Type, 2023-2033
9.2.1.2. Market size analysis, by Stage of Disease, 2023-2033
9.2.1.3. Market size analysis, by Route of Administration, 2023-2033
9.2.1.4. Market size analysis, by End-user, 2023-2033
9.2.2. UK
9.2.2.1. Market size analysis, by Type, 2023-2033
9.2.2.2. Market size analysis, by Stage of Disease, 2023-2033
9.2.2.3. Market size analysis, by Route of Administration, 2023-2033
9.2.2.4. Market size analysis, by End-user, 2023-2033
9.2.3. France
9.2.3.1. Market size analysis, by Type, 2023-2033
9.2.3.2. Market size analysis, by Stage of Disease, 2023-2033
9.2.3.3. Market size analysis, by Route of Administration, 2023-2033
9.2.3.4. Market size analysis, by End-user, 2023-2033
9.2.4. Spain
9.2.4.1. Market size analysis, by Type, 2023-2033
9.2.4.2. Market size analysis, by Stage of Disease, 2023-2033
9.2.4.3. Market size analysis, by Route of Administration, 2023-2033
9.2.4.4. Market size analysis, by End-user, 2023-2033
9.2.5. Italy
9.2.5.1. Market size analysis, by Type, 2023-2033
9.2.5.2. Market size analysis, by Stage of Disease, 2023-2033
9.2.5.3. Market size analysis, by Route of Administration, 2023-2033
9.2.5.4. Market size analysis, by End-user, 2023-2033
9.2.6. Rest of Europe
9.2.6.1. Market size analysis, by Type, 2023-2033
9.2.6.2. Market size analysis, by Stage of Disease, 2023-2033
9.2.6.3. Market size analysis, by Route of Administration, 2023-2033
9.2.6.4. Market size analysis, by End-user, 2023-2033
9.2.7. Research Dive Exclusive Insights
9.2.7.1. Market attractiveness
9.2.7.2. Competition heatmap
9.3. Asia-Pacific
9.3.1. China
9.3.1.1. Market size analysis, by Type, 2023-2033
9.3.1.2. Market size analysis, by Stage of Disease, 2023-2033
9.3.1.3. Market size analysis, by Route of Administration, 2023-2033
9.3.1.4. Market size analysis, by End-user, 2023-2033
9.3.2. Japan
9.3.2.1. Market size analysis, by Type, 2023-2033
9.3.2.2. Market size analysis, by Stage of Disease, 2023-2033
9.3.2.3. Market size analysis, by Route of Administration, 2023-2033
9.3.2.4. Market size analysis, by End-user, 2023-2033
9.3.3. India
9.3.3.1. Market size analysis, by Type, 2023-2033
9.3.3.2. Market size analysis, by Stage of Disease, 2023-2033
9.3.3.3. Market size analysis, by Route of Administration, 2023-2033
9.3.3.4. Market size analysis, by End-user, 2023-2033
9.3.4. Australia
9.3.4.1. Market size analysis, by Type, 2023-2033
9.3.4.2. Market size analysis, by Stage of Disease, 2023-2033
9.3.4.3. Market size analysis, by Route of Administration, 2023-2033
9.3.4.4. Market size analysis, by End-user, 2023-2033
9.3.5. South Korea
9.3.5.1. Market size analysis, by Type, 2023-2033
9.3.5.2. Market size analysis, by Stage of Disease, 2023-2033
9.3.5.3. Market size analysis, by Route of Administration, 2023-2033
9.3.5.4. Market size analysis, by End-user, 2023-2033
9.3.6. Rest of Asia-Pacific
9.3.6.1. Market size analysis, by Type, 2023-2033
9.3.6.2. Market size analysis, by Stage of Disease, 2023-2033
9.3.6.3. Market size analysis, by Route of Administration, 2023-2033
9.3.6.4. Market size analysis, by End-user, 2023-2033
9.3.7. Research Dive Exclusive Insights
9.3.7.1. Market attractiveness
9.3.7.2. Competition heatmap
9.4. LAMEA
9.4.1. Brazil
9.4.1.1. Market size analysis, by Type, 2023-2033
9.4.1.2. Market size analysis, by Stage of Disease, 2023-2033
9.4.1.3. Market size analysis, by Route of Administration, 2023-2033
9.4.1.4. Market size analysis, by End-user, 2023-2033
9.4.2. UAE
9.4.2.1. Market size analysis, by Type, 2023-2033
9.4.2.2. Market size analysis, by Stage of Disease, 2023-2033
9.4.2.3. Market size analysis, by Route of Administration, 2023-2033
9.4.2.4. Market size analysis, by End-user, 2023-2033
9.4.3. Saudi Arabia
9.4.3.1. Market size analysis, by Type, 2023-2033
9.4.3.2. Market size analysis, by Stage of Disease, 2023-2033
9.4.3.3. Market size analysis, by Route of Administration, 2023-2033
9.4.3.4. Market size analysis, by End-user, 2023-2033
9.4.4. South Africa
9.4.4.1. Market size analysis, by Type, 2023-2033
9.4.4.2. Market size analysis, by Stage of Disease, 2023-2033
9.4.4.3. Market size analysis, by Route of Administration, 2023-2033
9.4.4.4. Market size analysis, by End-user, 2023-2033
9.4.5. Rest of LAMEA
9.4.5.1. Market size analysis, by Type, 2023-2033
9.4.5.2. Market size analysis, by Stage of Disease, 2023-2033
9.4.5.3. Market size analysis, by Route of Administration, 2023-2033
9.4.5.4. Market size analysis, by End-user, 2023-2033
9.4.6. Research Dive Exclusive Insights
9.4.6.1. Market attractiveness
9.4.6.2. Competition heatmap
10. Competitive Landscape
10.1. Top winning strategies, 2022
10.1.1. By strategy
10.1.2. By year
10.2. Strategic overview
10.3. Market share analysis, 2022
11. Company Profiles
11.1. REGENXBIO Inc.
11.1.1. Overview
11.1.2. Business segments
11.1.3. Industry portfolio
11.1.4. Financial performance
11.1.5. Recent developments
11.1.6. SWOT analysis
11.2. Panoptica
11.2.1. Overview
11.2.2. Business segments
11.2.3. Industry portfolio
11.2.4. Financial performance
11.2.5. Recent developments
11.2.6. SWOT analysis
11.3. Regeneron
11.3.1. Overview
11.3.2. Business segments
11.3.3. Industry portfolio
11.3.4. Financial performance
11.3.5. Recent developments
11.3.6. SWOT analysis
11.4. Bausch Health Companies Inc.
11.4.1. Overview
11.4.2. Business segments
11.4.3. Industry portfolio
11.4.4. Financial performance
11.4.5. Recent developments
11.4.6. SWOT analysis
11.5. Novartis AG
11.5.1. Overview
11.5.2. Business segments
11.5.3. Industry portfolio
11.5.4. Financial performance
11.5.5. Recent developments
11.5.6. SWOT analysis
11.6. F. Hoffmann-La Roche AG
11.6.1. Overview
11.6.2. Business segments
11.6.3. Industry portfolio
11.6.4. Financial performance
11.6.5. Recent developments
11.6.6. SWOT analysis
11.7. Pfizer Inc.
11.7.1. Overview
11.7.2. Business segments
11.7.3. Industry portfolio
11.7.4. Financial performance
11.7.5. Recent developments
11.7.6. SWOT analysis
11.8. Thermo Fischer Scientific, Inc.
11.8.1. Overview
11.8.2. Business segments
11.8.3. Industry portfolio
11.8.4. Financial performance
11.8.5. Recent developments
11.8.6. SWOT analysis
11.9. Astellas Pharma Inc.
11.9.1. Overview
11.9.2. Business segments
11.9.3. Industry portfolio
11.9.4. Financial performance
11.9.5. Recent developments
11.9.6. SWOT analysis
11.10. Aerie Pharmaceutical Inc.
11.10.1. Overview
11.10.2. Business segments
11.10.3. Industry portfolio
11.10.4. Financial performance
11.10.5. Recent developments
11.10.6. SWOT analysis
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com

