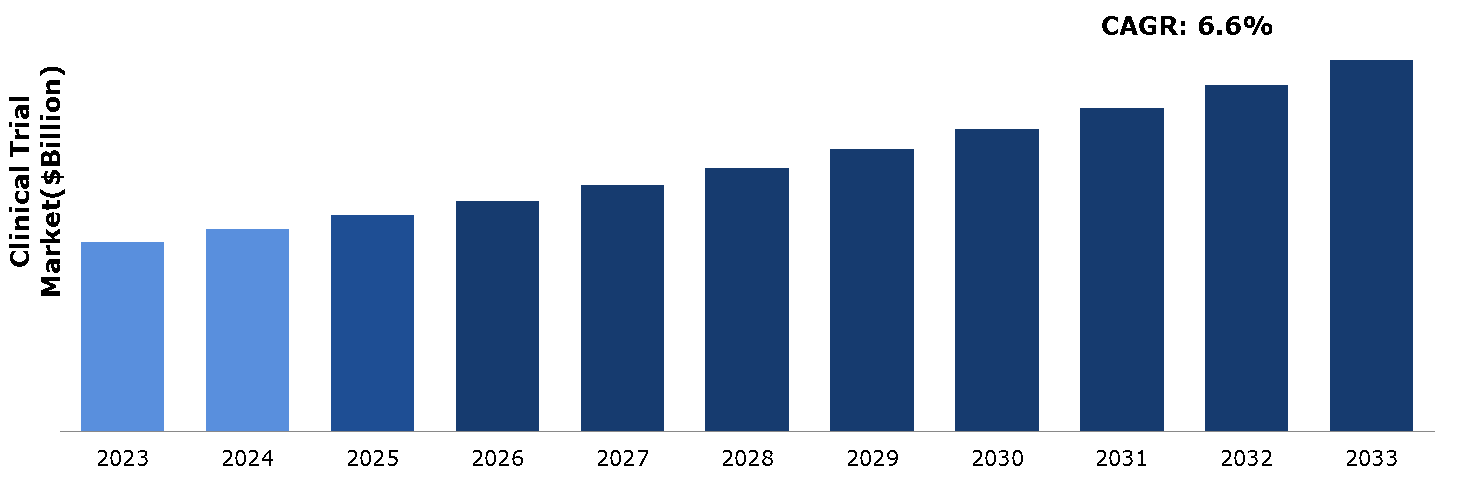Clinical Trials Market Size Share Competitive Landscape And Trend Analysis Report Report
RA01971
Clinical Trials Market Size, Share, Competitive Landscape, and Trend Analysis Report by Phase, Indication, Study Design, Service Type, and Region: Global Opportunity Analysis and Industry Forecast, 2024-2033
The clinical trial market was valued at $79.94 billion in 2023 and is estimated to reach $150.35 billion by 2033, exhibiting a CAGR of 6.6% from 2024 to 2033.
Overview of Clinical Trial
Clinical trials are crucial research studies that assess medical, surgical, or behavioral interventions in humans. They serve as the primary method for determining the safety and efficacy of new treatments or preventive measures, such as medications, diets, or medical devices like pacemakers. Typically, these trials aim to establish whether a new treatment outperforms existing options or offers fewer adverse effects. Additionally, clinical research practices facilitate early disease detection methods, sometimes before symptoms. Furthermore, it seeks to enhance the quality of life for those affected with severe illnesses or chronic health conditions. Overall, clinical trials play a major role in advancing medical knowledge and improving patient outcomes across various health concerns.
Key Takeaways
- The clinical trial market study covers 20 countries. The research includes a segment analysis of each country in terms of value ($billion) for the projected period from 2024 to 2033.
- More than 1,500 product literatures, industry releases, annual reports, and other such documents of major clinical trial industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach intends to provide a balanced view of global markets and assist stakeholders in making informed decisions in order to achieve their most ambitious growth objectives.
Industry Trends
- In February 2024, The National Cancer Institute (NCI), a part of the National Institutes of Health, launched a new initiative to address the significant drop in participation in NCI-funded cancer clinical trials, exacerbated by staffing shortages worsened during the COVID-19 pandemic. This initiative targets clinical trials within key NCI networks: the National Clinical Trials Network, the Experimental Therapeutics Clinical Trials Network, and the Community Oncology Research Program (NCORP). By focusing support on these networks, the NCI aims to bolster participation and ensure the advancement of critical research in cancer treatment and care nationwide.
- In November 2022, Castor, a company in decentralized and hybrid clinical trial technology, introduced a unique solution aimed at streamlining post-marketing trials. This innovation promises to simplify trial complexities by extending global reach, integrating real-world data (RWD), and automating essential processes. By leveraging these advancements, Castor's latest offering minimizes trial costs by up to 30% and remarkably shortens deployment timelines to an average of just four weeks. This strategic move is set to revolutionize post-marketing clinical research, making it more efficient, cost-effective, and accessible on a global scale.
Key Market Dynamics
The surging penetration of personalized medicine and the increasing demand for Contract Research Organizations (CROs) to conduct research activities are anticipated to significantly boost the demand for clinical trials. As personalized medicine provides specific treatments to individual patient profiles, it necessitates extensive and specialized clinical research to develop and validate these customized therapies. Consequently, the reliance on CROs for their expertise and resources in managing complex clinical trials is expected to rise.
Moreover, ensuring the integrity, accuracy, and privacy of data collected during clinical trials is critical yet challenging. This necessitates advanced systems and strict compliance with data protection regulations. Effective data management involves implementing comprehensive measures to protect against data breaches and inaccuracies, which can compromise trial results and patient privacy. These challenges significantly hamper the growth of clinical trials, as maintaining high standards of data protection requires substantial resources and expertise.
However, digitization in biomedical research is driving overall market growth by incorporating advanced technologies like Electronic Data Capture (EDC). EDC streamlines data collection and patient data management, significantly reducing monitoring costs and accelerating clinical trial timelines. By enabling real-time data access and analysis, EDC enhances the efficiency and accuracy of clinical trials, ultimately fostering growth in this sector. This technological advancement not only improves data management but also ensures faster decision-making, which is crucial for the timely development and approval of new treatments.
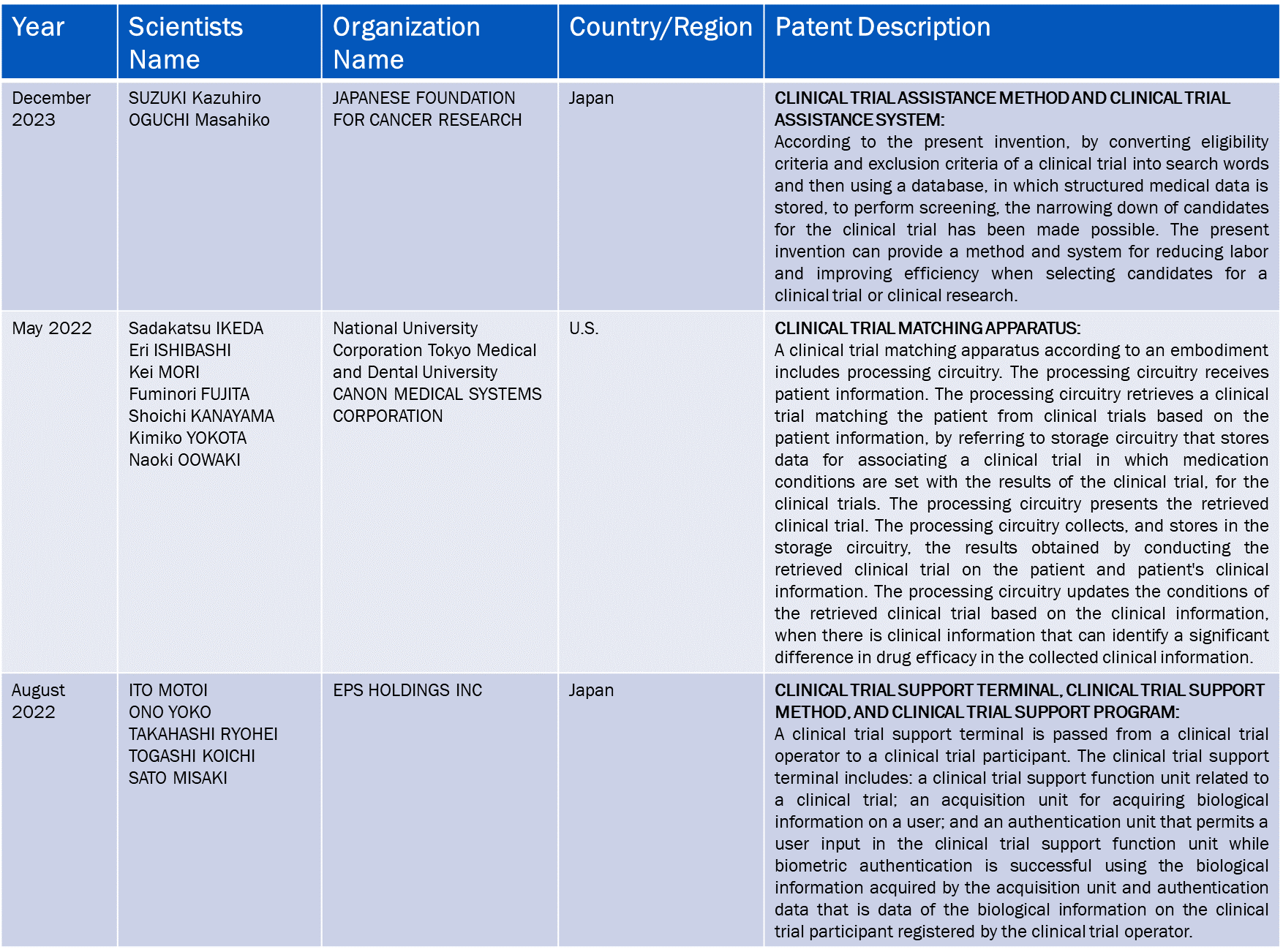
Patent Analysis
Market Segmentation
The clinical trial market is segmented into phase, indication, study design, service type, and region. On the basis of phase, the market is divided into Phase I, Phase II, Phase III, and Phase IV. As per indication, the market is segregated into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular, and others. Based on study design the market is segmented into interventional trials, observational trials, and expanded access trials. On the basis of service type the market is segmented into laboratory services, clinical trial data management services, patient recruitment, protocol designing, and others. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Country Market Outlook
The clinical trial market in India is experiencing significant growth driven by multiple factors. The country has a large and diverse patient population, a cost-effective operational environment, and a pool of skilled medical professionals, making it an attractive destination for clinical research. Regulatory reforms, such as the introduction of the New Drugs and Clinical Trials Rules in 2019, have streamlined the approval process, enhancing both efficiency and transparency. Additionally, the rise in healthcare infrastructure and increasing investments from global pharmaceutical companies further bolster this growth. These factors collectively position India as a key player in the global clinical trial industry, offering a competitive edge in terms of cost and quality.
In October 2023, Roche Pharma India launched its clinical trial excellence project to enhance the capabilities of public health institutions in conducting clinical trials and drug research. Aiming to transform these government hospitals into centers of excellence, the initiative focuses on aligning them with global standards. In its first phase, Roche would partner with 10 government hospitals, providing training for research teams, improving processes, and digitizing dossier submission and review by Ethics Committees. These efforts are expected to elevate the quality of clinical research, emphasizing innovative treatments and patient safety.
Competitive Landscape
The major players operating in the clinical trial market include Charles River Laboratories International, Inc., Pharmaceutical Product Development, inc. (Thermo Fisher Scientific, Inc.), Syneos Health, ICON plc, PAREXEL International Corporation, IQVIA, Chiltern International Ltd (Laboratory Corporation of America), SGS SA, Wuxi AppTec, Inc., and Eli Lilly and Company. Other players in the clinical trial market include Roche Pharma and Novo Nordisk A/S.
Recent Key Strategies and Developments
In February 2024, Denver-based PCM Trials, an independent provider of mobile research nurse visits for decentralized clinical trials (DCTs), acquired EmVenio Research, a Durham, North Carolina-based provider of community-based clinical trial sites with mobile research units. This immediate acquisition enhances PCM Trials' and EmVenio's capabilities to recruit and retain diverse populations for clinical research, crucial for regulatory approval. By combining strengths, PCM Trials gains access to EmVenio's site network, which focuses on underrepresented patient populations in hard-to-reach communities, allowing flexible collaboration with sponsors to meet study goals and deliver critical study data.
Key Sources Referred
- European Medicines Agency (EMA)
- CDER
- AstraZeneca
- Castor
- Roche Pharma
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the clinical trial market segments, current trends, estimations, and dynamics of the clinical trial market analysis from 2023 to 2033 to identify the prevailing clinical trial market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the clinical trial market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global clinical trial market statistics.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global clinical trial market trends, key players, market segments, application areas, and market growth strategies.
The market study comprises of all latest technological advancements, including the latest market development by major players operating in the market. Furthermore, the study also comprises detailed patent analysis.
| Aspect | Particulars |
| Historical Market Estimations | 2021-2022 |
| Base Year for Market Estimation | 2023 |
| Forecast Timeline for Market Projection | 2024-2033 |
| Geographical Scope | North America, Europe, Asia-Pacific, and LAMEA |
| Segmentation by Phase |
|
| Segmentation by Indication |
|
| Segmentation by Study Design |
|
| Segmentation by Service Type |
|
| Key Companies Profiled |
|
1. Research Methodology
1.1. Desk Research
1.2. Real time insights and validation
1.3. Forecast model
1.4. Assumptions and forecast parameters
1.5. Market size estimation
1.5.1. Top-down approach
1.5.2. Bottom-up approach
2. Report Scope
2.1. Market definition
2.2. Key objectives of the study
2.3. Market segmentation
3. Executive Summary
4. Market Overview
4.1. Introduction
4.2. Growth impact forces
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunities
4.3. Market value chain analysis
4.3.1. List of service provider
4.4. Innovation & sustainability matrices
4.4.1. Technology matrix
4.4.2. Patent matrix
4.4.3. Regulatory matrix
4.5. Porter’s five forces analysis
4.5.1. Bargaining power of suppliers
4.5.2. Bargaining power of consumers
4.5.3. Threat of substitutes
4.5.4. Threat of new entrants
4.5.5. Competitive Rivalry Intensity
4.6. PESTLE analysis
4.6.1. Political
4.6.2. Economical
4.6.3. Social
4.6.4. Technological
4.6.5. Legal
4.6.6. Environmental
5. Clinical Trial Market Analysis, by Phase
5.1. Overview
5.2. Phase I
5.2.1. Definition, key trends, growth factors, and opportunities
5.2.2. Market size analysis, by region, 2023-2033
5.2.3. Market share analysis, by country, 2023-2033
5.3. Phase II
5.3.1. Definition, key trends, growth factors, and opportunities
5.3.2. Market size analysis, by region, 2023-2033
5.3.3. Market share analysis, by country, 2023-2033
5.4. Phase III
5.4.1. Definition, key trends, growth factors, and opportunities
5.4.2. Market size analysis, by region, 2023-2033
5.4.3. Market share analysis, by country, 2023-2033
5.5. Phase IV
5.5.1. Definition, key trends, growth factors, and opportunities
5.5.2. Market size analysis, by region, 2023-2033
5.5.3. Market share analysis, by country, 2023-2033
5.6. Research Dive Exclusive Insights
5.6.1. Market attractiveness
5.6.2. Competition heatmap
6. Clinical Trial Market Analysis, by Indication
6.1. Overview
6.2. Autoimmune/Inflammation
6.2.1. Definition, key trends, growth factors, and opportunities
6.2.2. Market size analysis, by region, 2023-2033
6.2.3. Market share analysis, by country, 2023-2033
6.3. Pain Management
6.3.1. Definition, key trends, growth factors, and opportunities
6.3.2. Market size analysis, by region, 2023-2033
6.3.3. Market share analysis, by country, 2023-2033
6.4. Oncology
6.4.1. Definition, key trends, growth factors, and opportunities
6.4.2. Market size analysis, by region, 2023-2033
6.4.3. Market share analysis, by country, 2023-2033
6.5. CNS Condition
6.5.1. Definition, key trends, growth factors, and opportunities
6.5.2. Market size analysis, by region, 2023-2033
6.5.3. Market share analysis, by country, 2023-2033
6.6. Diabetes
6.6.1. Definition, key trends, growth factors, and opportunities
6.6.2. Market size analysis, by region, 2023-2033
6.6.3. Market share analysis, by country, 2023-2033
6.7. Obesity
6.7.1. Definition, key trends, growth factors, and opportunities
6.7.2. Market size analysis, by region, 2023-2033
6.7.3. Market share analysis, by country, 2023-2033
6.8. Cardiovascular
6.8.1. Definition, key trends, growth factors, and opportunities
6.8.2. Market size analysis, by region, 2023-2033
6.8.3. Market share analysis, by country, 2023-2033
6.9. Others
6.9.1. Definition, key trends, growth factors, and opportunities
6.9.2. Market size analysis, by region, 2023-2033
6.9.3. Market share analysis, by country, 2023-2033
6.10. Research Dive Exclusive Insights
6.10.1. Market attractiveness
6.10.2. Competition heatmap
7. Clinical Trial Market Analysis, by Study Design
7.1. Overview
7.2. Interventional Trials
7.2.1. Definition, key trends, growth factors, and opportunities
7.2.2. Market size analysis, by region, 2023-2033
7.2.3. Market share analysis, by country, 2023-2033
7.3. Observational Trials
7.3.1. Definition, key trends, growth factors, and opportunities
7.3.2. Market size analysis, by region, 2023-2033
7.3.3. Market share analysis, by country, 2023-2033
7.4. Expanded Access Trials
7.4.1. Definition, key trends, growth factors, and opportunities
7.4.2. Market size analysis, by region, 2023-2033
7.4.3. Market share analysis, by country, 2023-2033
7.5. Research Dive Exclusive Insights
7.5.1. Market attractiveness
7.5.2. Competition heatmap
8. Clinical Trial Market Analysis, by Service Type
8.1. Overview
8.2. Laboratory Services
8.2.1. Definition, key trends, growth factors, and opportunities
8.2.2. Market size analysis, by region, 2023-2033
8.2.3. Market share analysis, by country, 2023-2033
8.3. Clinical Trial Data Management Services
8.3.1. Definition, key trends, growth factors, and opportunities
8.3.2. Market size analysis, by region, 2023-2033
8.3.3. Market share analysis, by country, 2023-2033
8.4. Patient Recruitment
8.4.1. Definition, key trends, growth factors, and opportunities
8.4.2. Market size analysis, by region, 2023-2033
8.4.3. Market share analysis, by country, 2023-2033
8.5. Protocol Designing
8.5.1. Definition, key trends, growth factors, and opportunities
8.5.2. Market size analysis, by region, 2023-2033
8.5.3. Market share analysis, by country, 2023-2033
8.6. Others
8.6.1. Definition, key trends, growth factors, and opportunities
8.6.2. Market size analysis, by region, 2023-2033
8.6.3. Market share analysis, by country, 2023-2033
8.7. Research Dive Exclusive Insights
8.7.1. Market attractiveness
8.7.2. Competition heatmap
9. Clinical Trial Market, by Region
9.1. North America
9.1.1. U.S.
9.1.1.1. Market size analysis, by Phase, 2023-2033
9.1.1.2. Market size analysis, by Indication, 2023-2033
9.1.1.3. Market size analysis, by Study Design, 2023-2033
9.1.1.4. Market size analysis, by Service Type, 2023-2033
9.1.2. Canada
9.1.2.1. Market size analysis, by Phase, 2023-2033
9.1.2.2. Market size analysis, by Indication, 2023-2033
9.1.2.3. Market size analysis, by Study Design, 2023-2033
9.1.2.4. Market size analysis, by Service Type, 2023-2033
9.1.3. Mexico
9.1.3.1. Market size analysis, by Phase, 2023-2033
9.1.3.2. Market size analysis, by Indication, 2023-2033
9.1.3.3. Market size analysis, by Study Design, 2023-2033
9.1.3.4. Market size analysis, by Service Type, 2023-2033
9.1.4. Research Dive Exclusive Insights
9.1.4.1. Market attractiveness
9.1.4.2. Competition heatmap
9.2. Europe
9.2.1. Germany
9.2.1.1. Market size analysis, by Phase, 2023-2033
9.2.1.2. Market size analysis, by Indication, 2023-2033
9.2.1.3. Market size analysis, by Study Design, 2023-2033
9.2.1.4. Market size analysis, by Service Type, 2023-2033
9.2.2. UK
9.2.2.1. Market size analysis, by Phase, 2023-2033
9.2.2.2. Market size analysis, by Indication, 2023-2033
9.2.2.3. Market size analysis, by Study Design, 2023-2033
9.2.2.4. Market size analysis, by Service Type, 2023-2033
9.2.3. France
9.2.3.1. Market size analysis, by Phase, 2023-2033
9.2.3.2. Market size analysis, by Indication, 2023-2033
9.2.3.3. Market size analysis, by Study Design, 2023-2033
9.2.3.4. Market size analysis, by Service Type, 2023-2033
9.2.4. Spain
9.2.4.1. Market size analysis, by Phase, 2023-2033
9.2.4.2. Market size analysis, by Indication, 2023-2033
9.2.4.3. Market size analysis, by Study Design, 2023-2033
9.2.4.4. Market size analysis, by Service Type, 2023-2033
9.2.5. Italy
9.2.5.1. Market size analysis, by Phase, 2023-2033
9.2.5.2. Market size analysis, by Indication, 2023-2033
9.2.5.3. Market size analysis, by Study Design, 2023-2033
9.2.5.4. Market size analysis, by Service Type, 2023-2033
9.2.6. Rest of Europe
9.2.6.1. Market size analysis, by Phase, 2023-2033
9.2.6.2. Market size analysis, by Indication, 2023-2033
9.2.6.3. Market size analysis, by Study Design, 2023-2033
9.2.6.4. Market size analysis, by Service Type, 2023-2033
9.2.7. Research Dive Exclusive Insights
9.2.7.1. Market attractiveness
9.2.7.2. Competition heatmap
9.3. Asia-Pacific
9.3.1. China
9.3.1.1. Market size analysis, by Phase, 2023-2033
9.3.1.2. Market size analysis, by Indication, 2023-2033
9.3.1.3. Market size analysis, by Study Design, 2023-2033
9.3.1.4. Market size analysis, by Service Type, 2023-2033
9.3.2. Japan
9.3.2.1. Market size analysis, by Phase, 2023-2033
9.3.2.2. Market size analysis, by Indication, 2023-2033
9.3.2.3. Market size analysis, by Study Design, 2023-2033
9.3.2.4. Market size analysis, by Service Type, 2023-2033
9.3.3. India
9.3.3.1. Market size analysis, by Phase, 2023-2033
9.3.3.2. Market size analysis, by Indication, 2023-2033
9.3.3.3. Market size analysis, by Study Design, 2023-2033
9.3.3.4. Market size analysis, by Service Type, 2023-2033
9.3.4. Australia
9.3.4.1. Market size analysis, by Phase, 2023-2033
9.3.4.2. Market size analysis, by Indication, 2023-2033
9.3.4.3. Market size analysis, by Study Design, 2023-2033
9.3.4.4. Market size analysis, by Service Type, 2023-2033
9.3.5. South Korea
9.3.5.1. Market size analysis, by Phase, 2023-2033
9.3.5.2. Market size analysis, by Indication, 2023-2033
9.3.5.3. Market size analysis, by Study Design, 2023-2033
9.3.5.4. Market size analysis, by Service Type, 2023-2033
9.3.6. Rest of Asia-Pacific
9.3.6.1. Market size analysis, by Phase, 2023-2033
9.3.6.2. Market size analysis, by Indication, 2023-2033
9.3.6.3. Market size analysis, by Study Design, 2023-2033
9.3.6.4. Market size analysis, by Service Type, 2023-2033
9.3.7. Research Dive Exclusive Insights
9.3.7.1. Market attractiveness
9.3.7.2. Competition heatmap
9.4. LAMEA
9.4.1. Brazil
9.4.1.1. Market size analysis, by Phase, 2023-2033
9.4.1.2. Market size analysis, by Indication, 2023-2033
9.4.1.3. Market size analysis, by Study Design, 2023-2033
9.4.1.4. Market size analysis, by Service Type, 2023-2033
9.4.2. UAE
9.4.2.1. Market size analysis, by Phase, 2023-2033
9.4.2.2. Market size analysis, by Indication, 2023-2033
9.4.2.3. Market size analysis, by Study Design, 2023-2033
9.4.2.4. Market size analysis, by Service Type, 2023-2033
9.4.3. Saudi Arabia
9.4.3.1. Market size analysis, by Phase, 2023-2033
9.4.3.2. Market size analysis, by Indication, 2023-2033
9.4.3.3. Market size analysis, by Study Design, 2023-2033
9.4.3.4. Market size analysis, by Service Type, 2023-2033
9.4.4. South Africa
9.4.4.1. Market size analysis, by Phase, 2023-2033
9.4.4.2. Market size analysis, by Indication, 2023-2033
9.4.4.3. Market size analysis, by Study Design, 2023-2033
9.4.4.4. Market size analysis, by Service Type, 2023-2033
9.4.5. Rest of LAMEA
9.4.5.1. Market size analysis, by Phase, 2023-2033
9.4.5.2. Market size analysis, by Indication, 2023-2033
9.4.5.3. Market size analysis, by Study Design, 2023-2033
9.4.5.4. Market size analysis, by Service Type, 2023-2033
9.4.6. Research Dive Exclusive Insights
9.4.6.1. Market attractiveness
9.4.6.2. Competition heatmap
10. Competitive Landscape
10.1. Top winning strategies, 2022
10.1.1. By strategy
10.1.2. By year
10.2. Strategic overview
10.3. Market share analysis, 2022
11. Company Profiles
11.1. Charles River Laboratories International, Inc.
11.1.1. Overview
11.1.2. Business segments
11.1.3. Product portfolio
11.1.4. Financial performance
11.1.5. Recent developments
11.1.6. SWOT analysis
11.2. Pharmaceutical Product Development, Inc. (Thermo Fisher Scientific, Inc.)
11.2.1. Overview
11.2.2. Business segments
11.2.3. Product portfolio
11.2.4. Financial performance
11.2.5. Recent developments
11.2.6. SWOT analysis
11.3. Syneos Health
11.3.1. Overview
11.3.2. Business segments
11.3.3. Product portfolio
11.3.4. Financial performance
11.3.5. Recent developments
11.3.6. SWOT analysis
11.4. ICON plc
11.4.1. Overview
11.4.2. Business segments
11.4.3. Product portfolio
11.4.4. Financial performance
11.4.5. Recent developments
11.4.6. SWOT analysis
11.5. PAREXEL International Corporation
11.5.1. Overview
11.5.2. Business segments
11.5.3. Product portfolio
11.5.4. Financial performance
11.5.5. Recent developments
11.5.6. SWOT analysis
11.6. IQVIA
11.6.1. Overview
11.6.2. Business segments
11.6.3. Product portfolio
11.6.4. Financial performance
11.6.5. Recent developments
11.6.6. SWOT analysis
11.7. Chiltern International Ltd. (Laboratory Corporation of America)
11.7.1. Overview
11.7.2. Business segments
11.7.3. Product portfolio
11.7.4. Financial performance
11.7.5. Recent developments
11.7.6. SWOT analysis
11.8. SGS SA
11.8.1. Overview
11.8.2. Business segments
11.8.3. Product portfolio
11.8.4. Financial performance
11.8.5. Recent developments
11.8.6. SWOT analysis
11.9. Wuxi AppTec, Inc.
11.9.1. Overview
11.9.2. Business segments
11.9.3. Product portfolio
11.9.4. Financial performance
11.9.5. Recent developments
11.9.6. SWOT analysis
11.10. Eli Lilly and Company
11.10.1. Overview
11.10.2. Business segments
11.10.3. Product portfolio
11.10.4. Financial performance
11.10.5. Recent developments
11.10.6. SWOT analysis
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com