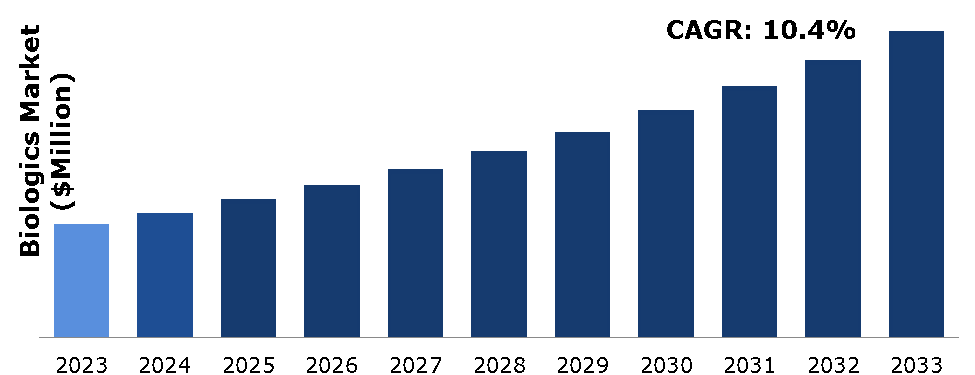Biologics Market Size Share Competitive Landscape And Trend Analysis Report Report
RA01160
Biologics Market Size, Share, Competitive Landscape, and Trend Analysis Report by Product, Application, Source, and Region: Global Opportunity Analysis and Industry Forecast, 2024-2033
The biologics market was valued at $540,346.62 million in 2023 and is estimated to reach $1,460,572.05 million by 2033 exhibiting a CAGR of 10.4% from 2024 to 2033
Market Introduction and Definition
Biologics are a diverse category of products derived from living organisms, including humans, animals, and microorganisms, used in the prevention, treatment, or cure of diseases and medical conditions. Unlike traditional chemically synthesized drugs, biologics are composed of complex molecules or mixtures of molecules, such as proteins, nucleic acids, or cells and tissues. They include a wide range of products such as vaccines, blood and blood components, gene therapies, tissues, recombinant therapeutic proteins, and monoclonal antibodies. The production of biologics involves advanced biotechnological methods, including recombinant DNA technology and hybridoma technology.
Biologics are often highly specific in their action, targeting particular disease pathways or mechanisms at a molecular level, due to their complexity and the intricacies of their production processes. This specificity can lead to increased efficacy and fewer side effects compared to conventional drugs.
Key Takeaways
- The biologics market study covers 20 countries. The research includes a segment analysis of each country in terms of value ($million) for the projected period from 2024 to 2033.
- More than 1,500 product literatures, industry releases, annual reports, and other such documents of major energy storage system industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach intends to provide a balanced view of global markets and assist stakeholders in making informed decisions in order to achieve their most ambitious growth objectives.
Industry Trends
- In May 2022, Biocon Biologics and Viatris (formerly Mylan) introduced the cancer medication Bevacizumab in Canada under the brand name Abevmy. Abevmy was collaboratively developed by Biocon Biologics and Viatris.
- In February 2022, according to the World Health Organization, there has been a significant increase in cancer prevalence, particularly in the most common types of cancer in terms of both new cases and associated fatalities. Breast cancer emerged as the leading type with 2.26 million new cases, closely followed by lung cancer at 2.21 million cases. Colon and rectum cancer accounted for 1.93 million cases, while prostate cancer and non-melanoma skin cancer contributed 1.41 million and 1.20 million cases, respectively. Stomach cancer also stood out, with 1.09 million new cases reported in 2022.
- In February 2022, the Health Minister of Tamil Nadu, India, announced that the government is actively developing a policy aimed at detecting 66% of cancer cases in the initial and intermediate stages by 2030, to ensure timely and adequate treatment.
Key Market Dynamics
As the global population ages, the incidence of chronic diseases tends to increase. Aging is a significant risk factor for many chronic conditions such as cancer, diabetes, and rheumatoid arthritis. Chronic diseases are becoming more prevalent worldwide, not only in developed countries but also in emerging economies. Factors such as urbanization, sedentary lifestyles, and changing dietary habits contribute to this rise. Improved diagnostic techniques enable healthcare providers to better identify and diagnose chronic diseases at earlier stages. This leads to an increase in demand for effective treatments like biologics to manage these conditions and improve patient outcomes. Biologics offer targeted therapies that can often achieve better treatment outcomes compared to conventional treatments for chronic diseases. They can selectively block specific pathways involved in disease progression, providing more precise and personalized treatment options.
Developing a biologic typically involves extensive research to understand the biological mechanisms, identify suitable targets, and design the therapeutic molecule. This phase requires funding for employing researchers, procuring equipment, and conducting experiments. The complexity of biological systems often leads to prolonged R&D timelines and higher expenses. Biologics are typically produced using living cells or organisms, which necessitates specialized manufacturing facilities. Constructing and operating these facilities require substantial capital investment. These factors are anticipated to restrain the biologics market growth during the forecast period.
Rising advancements in manufacturing technologies like single-use bioreactors and continuous bioprocessing can significantly reduce production costs. Single-use bioreactors eliminate the need for cleaning and validation between batches, thus saving time and resources. Continuous bioprocessing allows for a more streamlined production process, reducing downtime and increasing overall efficiency. These cost savings can translate into lower prices for biologic drugs, making them more affordable for patients. By improving efficiency and scalability, these technological advancements enable biopharmaceutical companies to ramp up production capacity to meet growing demand. This is particularly crucial for biologics, which often have complex manufacturing processes and limited production capacity. These factors are anticipated to create opportunities in the biologics market during the forecast years.
Public Policies in the Global Biologics Market
Public policies for the biologics market play a crucial role in balancing innovation, accessibility, affordability, and safety.
- Regulatory Frameworks: Governments establish regulatory agencies (e.g., FDA in the U.S., EMA in the EU) to ensure the safety, efficacy, and quality of biologic products. These agencies set guidelines for the approval and post-market surveillance of biologics.
- Intellectual Property Protection: Governments grant patents to incentivize innovation in biologics. However, there is often a delicate balance between providing adequate protection for innovators and ensuring access to affordable biosimilars once patents expire. Policies like patent cliffs or compulsory licensing may be used to encourage competition and lower prices.
- Health Technology Assessment (HTA): Many countries conduct HTA to assess the value of biologics compared to existing treatments. HTA informs reimbursement decisions by considering factors like clinical efficacy, safety, cost-effectiveness, and societal impact.
- Patient Safety and Monitoring: Post-market surveillance systems are established to monitor the safety and efficacy of biologic products once they are in the market. Adverse event reporting and pharmacovigilance programs are essential components of these systems.
Market Segmentation
The biologics market is segmented into product, application, source, and region. On the basis of product, the market is divided into monoclonal antibodies, vaccines, recombinant hormones/proteins, cellular-based biologics, gene-based biologics, and other products. On the basis of application, the market is divided into cancer, infectious diseases, autoimmune diseases, and other applications. On the basis of source, the market is classified into microbial and mammalian. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional Market Outlook
The aging population in North America has contributed to the growing demand for biologics, as senior adults are more prone to chronic and age-related diseases. By 2030, it is projected that there will be over 78 million people aged 65 and older in the U.S., according to the U.S. Census Bureau. Chronic diseases such as cancer, autoimmune disorders, and diabetes are on the rise in North America, leading to an increase in demand for biologic drugs which offer targeted and effective treatments. According to the Centers for Disease Control and Prevention (CDC), chronic diseases account for 7 out of every 10 deaths in the U.S. The biotechnology industry in the U.S. alone contributes over $1 trillion to the economy annually, according to the Biotechnology Innovation Organization (BIO).
On September 11, 2023, Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) received approval from the U.S. Food and Drug Administration (FDA) for their supplemental Biologics License Application (COMIRNATY 2023-2024 Formulation) for individuals aged 12 and above. In addition, emergency use authorization has been granted for children aged 6 months to 11 years for the companies’ Omicron XBB.1.5-adapted monovalent COVID-19 vaccine.
Competitive Landscape
The major players operating in the biologics market include AbbVie Inc., Amgen Inc., Eli Lilly and Company, F. Hoffmann-La Roche AG, GlaxoSmithKline PLC, Johnson & Johnson, Merck & Co., Pfizer Inc., Sanofi SA, Bristol Myers Squibb, and others.
Recent Key Strategies and Developments
- On September 29, 2023, Biogen Inc. (Nasdaq: BIIB) announced that the U.S. Food and Drug Administration (FDA) had approved TOFIDENCE (tocilizumab-bavi) intravenous formulation, a biosimilar monoclonal antibody referencing ACTEMRA. The TOFIDENCE intravenous formulation is approved for the treatment of moderately to severely active rheumatoid arthritis, polyarticular juvenile idiopathic arthritis and systemic juvenile idiopathic arthritis.
- In April 2022, GSK, a British multinational pharmaceutical and biotechnology company, along with SK bioscience, filed a biologics license application for SKYCovione, a COVID-19 vaccine candidate based on recombinant proteins, with the Korean Ministry of Food and Drug Safety (KMFDS).
Key Sources Referred
- World Health Organization
- Biologics Investment & Trade Reports
- Pharmaceuticals and Medical Devices Agency (PMDA)
- D&B Hoovers
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the biologics market segments, current trends, estimations, and dynamics of the biologics market analysis from 2023 to 2033 to identify the prevailing biologics market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the biologics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global biologics market statistics.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
Apart from the points mentioned above, the report includes the analysis of the regional as well as global biologics trends, key players, market segments, application areas, and market growth strategies.
| Aspect | Particulars |
| Historical Market Estimations | 2021-2022 |
| Base Year for Market Estimation | 2023 |
| Forecast Timeline for Market Projection | 2024-2033 |
| Geographical Scope | North America, Europe, Asia-Pacific, and LAMEA |
| Segmentation by Product |
|
| Segmentation by Application |
Other |
| Segmentation by Source |
|
| Key Companies Profiled |
|
1. Research Methodology
1.1. Desk Research
1.2. Real time insights and validation
1.3. Forecast model
1.4. Assumptions and forecast parameters
1.5. Market size estimation
1.5.1. Top-down approach
1.5.2. Bottom-up approach
2. Report Scope
2.1. Market definition
2.2. Key objectives of the study
2.3. Market segmentation
3. Executive Summary
4. Market Overview
4.1. Introduction
4.2. Growth impact forces
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunities
4.3. Market value chain analysis
4.3.1. List of raw material suppliers
4.3.2. List of manufacturers
4.3.3. List of distributors
4.4. Innovation & sustainability matrices
4.4.1. Technology matrix
4.4.2. Regulatory matrix
4.5. Porter’s five forces analysis
4.5.1. Bargaining power of suppliers
4.5.2. Bargaining power of consumers
4.5.3. Threat of substitutes
4.5.4. Threat of new entrants
4.5.5. Competitive Rivalry Intensity
4.6. PESTLE analysis
4.6.1. Political
4.6.2. Economical
4.6.3. Social
4.6.4. Technological
4.6.5. Legal
4.6.6. Environmental
4.7. Impact of COVID-19 on Biologics Market
4.7.1. Pre-covid market scenario
4.7.2. Post-covid market scenario
5. Biologics Market Analysis, by Product
5.1. Overview
5.2. Monoclonal Antibodies
5.2.1. Definition, key trends, growth factors, and opportunities
5.2.2. Market size analysis, by region, 2022-2032
5.2.3. Market share analysis, by country, 2022-2032
5.3. Vaccines
5.3.1. Definition, key trends, growth factors, and opportunities
5.3.2. Market size analysis, by region, 2022-2032
5.3.3. Market share analysis, by country, 2022-2032
5.4. Recombinant Hormones/Proteins
5.4.1. Definition, key trends, growth factors, and opportunities
5.4.2. Market size analysis, by region, 2022-2032
5.4.3. Market share analysis, by country, 2022-2032
5.5. Cellular-Based Biologics
5.5.1. Definition, key trends, growth factors, and opportunities
5.5.2. Market size analysis, by region, 2022-2032
5.5.3. Market share analysis, by country, 2022-2032
5.6. Gene-Based Biologics
5.6.1. Definition, key trends, growth factors, and opportunities
5.6.2. Market size analysis, by region, 2022-2032
5.6.3. Market share analysis, by country, 2022-2032
5.7. Other
5.7.1. Definition, key trends, growth factors, and opportunities
5.7.2. Market size analysis, by region, 2022-2032
5.7.3. Market share analysis, by country, 2022-2032
5.8. Research Dive Exclusive Insights
5.8.1. Market attractiveness
5.8.2. Competition heatmap
6. Biologics Market Analysis, by Application
6.1. Overview
6.2. Cancer
6.2.1. Definition, key trends, growth factors, and opportunities
6.2.2. Market size analysis, by region, 2022-2032
6.2.3. Market share analysis, by country, 2022-2032
6.3. Infectious Diseases
6.3.1. Definition, key trends, growth factors, and opportunities
6.3.2. Market size analysis, by region, 2022-2032
6.3.3. Market share analysis, by country, 2022-2032
6.4. Autoimmune Diseases
6.4.1. Definition, key trends, growth factors, and opportunities
6.4.2. Market size analysis, by region, 2022-2032
6.4.3. Market share analysis, by country, 2022-2032
6.5. Others
6.5.1. Definition, key trends, growth factors, and opportunities
6.5.2. Market size analysis, by region, 2022-2032
6.5.3. Market share analysis, by country, 2022-2032
6.6. Research Dive Exclusive Insights
6.6.1. Market attractiveness
6.6.2. Competition heatmap
7. Biologics Market Analysis, by Source
7.1. Overview
7.2. Microbial
7.2.1. Definition, key trends, growth factors, and opportunities
7.2.2. Market size analysis, by region, 2022-2032
7.2.3. Market share analysis, by country, 2022-2032
7.3. Mammalian
7.3.1. Definition, key trends, growth factors, and opportunities
7.3.2. Market size analysis, by region, 2022-2032
7.3.3. Market share analysis, by country, 2022-2032
7.4. Research Dive Exclusive Insights
7.4.1. Market attractiveness
7.4.2. Competition heatmap
8. Biologics Market , by Region
8.1. North America
8.1.1. U.S.
8.1.1.1. Market size analysis, by Product, 2022-2032
8.1.1.2. Market size analysis, by Application, 2022-2032
8.1.1.3. Market size analysis, by Source, 2022-2032
8.1.2. Canada
8.1.2.1. Market size analysis, by Product, 2022-2032
8.1.2.2. Market size analysis, by Application, 2022-2032
8.1.2.3. Market size analysis, by Source, 2022-2032
8.1.3. Mexico
8.1.3.1. Market size analysis, by Product, 2022-2032
8.1.3.2. Market size analysis, by Application, 2022-2032
8.1.3.3. Market size analysis, by Source, 2022-2032
8.1.4. Research Dive Exclusive Insights
8.1.4.1. Market attractiveness
8.1.4.2. Competition heatmap
8.2. Europe
8.2.1. Germany
8.2.1.1. Market size analysis, by Product, 2022-2032
8.2.1.2. Market size analysis, by Application, 2022-2032
8.2.1.3. Market size analysis, by Source, 2022-2032
8.2.2. UK
8.2.2.1. Market size analysis, by Product, 2022-2032
8.2.2.2. Market size analysis, by Application, 2022-2032
8.2.2.3. Market size analysis, by Source, 2022-2032
8.2.3. France
8.2.3.1. Market size analysis, by Product, 2022-2032
8.2.3.2. Market size analysis, by Application, 2022-2032
8.2.3.3. Market size analysis, by Source, 2022-2032
8.2.4. Spain
8.2.4.1. Market size analysis, by Product, 2022-2032
8.2.4.2. Market size analysis, by Application, 2022-2032
8.2.4.3. Market size analysis, by Source, 2022-2032
8.2.5. Italy
8.2.5.1. Market size analysis, by Product, 2022-2032
8.2.5.2. Market size analysis, by Application, 2022-2032
8.2.5.3. Market size analysis, by Source, 2022-2032
8.2.6. Rest of Europe
8.2.6.1. Market size analysis, by Product, 2022-2032
8.2.6.2. Market size analysis, by Application, 2022-2032
8.2.6.3. Market size analysis, by Source, 2022-2032
8.2.7. Research Dive Exclusive Insights
8.2.7.1. Market attractiveness
8.2.7.2. Competition heatmap
8.3. Asia-Pacific
8.3.1. China
8.3.1.1. Market size analysis, by Product, 2022-2032
8.3.1.2. Market size analysis, by Application, 2022-2032
8.3.1.3. Market size analysis, by Source, 2022-2032
8.3.2. Japan
8.3.2.1. Market size analysis, by Product, 2022-2032
8.3.2.2. Market size analysis, by Application, 2022-2032
8.3.2.3. Market size analysis, by Source, 2022-2032
8.3.3. India
8.3.3.1. Market size analysis, by Product, 2022-2032
8.3.3.2. Market size analysis, by Application, 2022-2032
8.3.3.3. Market size analysis, by Source, 2022-2032
8.3.4. Australia
8.3.4.1. Market size analysis, by Product, 2022-2032
8.3.4.2. Market size analysis, by Application, 2022-2032
8.3.4.3. Market size analysis, by Source, 2022-2032
8.3.5. South Korea
8.3.5.1. Market size analysis, by Product, 2022-2032
8.3.5.2. Market size analysis, by Application, 2022-2032
8.3.5.3. Market size analysis, by Source, 2022-2032
8.3.6. Rest of Asia-Pacific
8.3.6.1. Market size analysis, by Product, 2022-2032
8.3.6.2. Market size analysis, by Application, 2022-2032
8.3.6.3. Market size analysis, by Source, 2022-2032
8.3.7. Research Dive Exclusive Insights
8.3.7.1. Market attractiveness
8.3.7.2. Competition heatmap
8.4. LAMEA
8.4.1. Brazil
8.4.1.1. Market size analysis, by Product, 2022-2032
8.4.1.2. Market size analysis, by Application, 2022-2032
8.4.1.3. Market size analysis, by Source, 2022-2032
8.4.2. UAE
8.4.2.1. Market size analysis, by Product, 2022-2032
8.4.2.2. Market size analysis, by Application, 2022-2032
8.4.2.3. Market size analysis, by Source, 2022-2032
8.4.3. Saudi Arabia
8.4.3.1. Market size analysis, by Product, 2022-2032
8.4.3.2. Market size analysis, by Application, 2022-2032
8.4.3.3. Market size analysis, by Source, 2022-2032
8.4.4. South Africa
8.4.4.1. Market size analysis, by Product, 2022-2032
8.4.4.2. Market size analysis, by Application, 2022-2032
8.4.4.3. Market size analysis, by Source, 2022-2032
8.4.5. Rest of LAMEA
8.4.5.1. Market size analysis, by Product, 2022-2032
8.4.5.2. Market size analysis, by Application, 2022-2032
8.4.5.3. Market size analysis, by Source, 2022-2032
8.4.6. Research Dive Exclusive Insights
8.4.6.1. Market attractiveness
8.4.6.2. Competition heatmap
9. Competitive Landscape
9.1. Top winning strategies, 2022
9.1.1. By strategy
9.1.2. By year
9.2. Strategic overview
9.3. Market share analysis, 2022
10. Company Profiles
10.1. AbbVie Inc.
10.1.1. Overview
10.1.2. Business segments
10.1.3. Product portfolio
10.1.4. Financial performance
10.1.5. Recent developments
10.1.6. SWOT analysis
10.2. Amgen Inc.
10.2.1. Overview
10.2.2. Business segments
10.2.3. Product portfolio
10.2.4. Financial performance
10.2.5. Recent developments
10.2.6. SWOT analysis
10.3. Eli Lilly and Company
10.3.1. Overview
10.3.2. Business segments
10.3.3. Product portfolio
10.3.4. Financial performance
10.3.5. Recent developments
10.3.6. SWOT analysis
10.4. F. Hoffmann-La Roche AG
10.4.1. Overview
10.4.2. Business segments
10.4.3. Product portfolio
10.4.4. Financial performance
10.4.5. Recent developments
10.4.6. SWOT analysis
10.5. GlaxoSmithKline PLC
10.5.1. Overview
10.5.2. Business segments
10.5.3. Product portfolio
10.5.4. Financial performance
10.5.5. Recent developments
10.5.6. SWOT analysis
10.6. Johnson & Johnson
10.6.1. Overview
10.6.2. Business segments
10.6.3. Product portfolio
10.6.4. Financial performance
10.6.5. Recent developments
10.6.6. SWOT analysis
10.7. Merck & Co.
10.7.1. Overview
10.7.2. Business segments
10.7.3. Product portfolio
10.7.4. Financial performance
10.7.5. Recent developments
10.7.6. SWOT analysis
10.8. Pfizer Inc.
10.8.1. Overview
10.8.2. Business segments
10.8.3. Product portfolio
10.8.4. Financial performance
10.8.5. Recent developments
10.8.6. SWOT analysis
10.9. Sanofi SA
10.9.1. Overview
10.9.2. Business segments
10.9.3. Product portfolio
10.9.4. Financial performance
10.9.5. Recent developments
10.9.6. SWOT analysis
10.10. Bristol Myers Squibb
10.10.1. Overview
10.10.2. Business segments
10.10.3. Product portfolio
10.10.4. Financial performance
10.10.5. Recent developments
10.10.6. SWOT analysis
Personalize this research
- Triangulate with your own data
- Request your format and definition
- Get a deeper dive on a specific application, geography, customer or competitor
- + 1-888-961-4454 Toll - Free
- support@researchdive.com